
·ÖĪö I£®£Ø1£©ŗĻ½šÓ²¶Č“óÓŚø÷³É·Ö£»
£Ø2£©ĪļÖŹµÄĮæÅضČC=$\frac{1000¦Ńw}{M}$mol/L£»øł¾ŻĻ”ŹĶĒ°ŗóŃĪĖįµÄĪļÖŹµÄĮæ²»±ä¼ĘĖćŠčŅŖÅØŃĪĖįĢå»ż£»Ń”ȔȯĮæĘæ¹ęøńÓ¦øƵČÓŚ»ņÉŌ“óÓŚÅäÖĘČÜŅŗĢå»ż£»
£Ø3£©øł¾ŻĢāÖŠĮ÷³ĢæÉÖŖ£¬²ā¶ØŗĻ½šŃłĘ·ÖŠĀĮµÄÖŹĮæ·ÖŹż£¬»¹ŠčŅŖ³ĘĮæŗĻ½šŃłĘ·µÄÖŹĮ棻
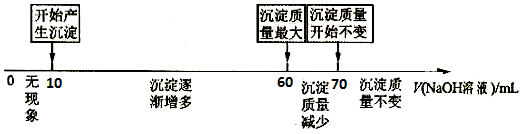
II£®£Ø3£©µ±¼ÓČė60”«70mlĒāŃõ»ÆÄĘČÜŅŗŹ±£¬³ĮµķµÄÖŹĮæ¼õŠ”£¬ŹĒĒāŃõ»ÆĀĮÓėĒāŃõ»ÆÄĘ·¢Éś·“Ӧɜ³ÉĮĖĘ«ĀĮĖįÄĘ£¬¾Ż“ĖŹéŠ“Ąė×Ó·½³ĢŹ½£»
£Ø4£©¼ÓČėNaOHČÜŅŗ50mL”«60mLĪŖAl£ØOH£©3ÓėNaOHµÄ·“Ó¦£¬æɼĘĖćAl3+µÄĪļÖŹµÄĮ棬Ōņ¼ÓČėNaOHČÜŅŗ10mL”«50mLĪŖAl3+ŗĶMg2+ĻūŗĵÄĢå»ż£¬øł¾Ż·½³ĢŹ½Mg2++2OH-=Mg£ØOH£©2”ż”¢Al3++3OH-=Al£ØOH£©3”żæÉÖŖAlŗĶMgµÄĪļÖŹµÄĮæÖ®±Č£¬½ų¶ųæɼĘĖćĆ¾µÄÖŹĮæ·ÖŹż£®
½ā“š ½ā£ŗI£®£Ø1£©Ć¾ĀĮŗĻ½šµÄÓ²¶Č±Č½šŹōĀĮµÄÓ²¶Č“󣬹Ź“š°øĪŖ£ŗ“ó£»
£Ø2£©ĪļÖŹµÄĮæÅضČC=$\frac{1000¦Ńw}{M}$mol/L=$\frac{1000”Į1.2”Į36.5%}{36.5}$=12mol/L£¬
Ļ”ŹĶĒ°ŗóŃĪĖįµÄĪļÖŹµÄĮæ²»±ä£¬ÅØŃĪĖįĢå»ż=$\frac{0.6mol/L”Į0.25l}{12mol/L}$=0.0125L=12.5ml£»Ń”ȔȯĮæĘæ¹ęøńÓ¦øƵČÓŚ»ņÉŌ“óÓŚÅäÖĘČÜŅŗĢå»ż£¬ŹµŃéŹŅƻӊ240mLČŻĮæĘ棬ӊ250mLČŻĮæĘ棬ĖłŅŌÓ¦øĆєȔ250mLČŻĮæĘ棬ĖłŠčµÄ²£Į§ŅĒĘ÷ÓŠ£ŗÉÕ±”¢²£Į§°ō”¢ĮæĶ²”¢250mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü£¬
¹Ź“š°øĪŖ£ŗ12.5£»250mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü£»
£Ø3£©×ĘÉÕµĆµ½µÄŹĒŃõ»ÆĆ¾£¬ĖłŅŌŅŖ¼ĘĖćŗĻ½šÖŠĆ¾µÄÖŹĮæ·ÖŹż£¬»¹ŠčŅŖ³ĘĮæŗĻ½šŃłĘ·µÄÖŹĮ棬
¹Ź“š°øĪŖ£ŗŗĻ½šŃłĘ·µÄÖŹĮ棻
II£®£Ø3£©µ±¼ÓČė60”«70mlĒāŃõ»ÆÄĘČÜŅŗŹ±£¬³ĮµķµÄÖŹĮæ¼õŠ”£¬ŹĒĒāŃõ»ÆĀĮÓėĒāŃõ»ÆÄĘ·¢Éś·“Ӧɜ³ÉĮĖĘ«ĀĮĖįÄĘ£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗAl£ØOH£©3+2OH-=2 AlO2-+H2O£¬
¹Ź“š°øĪŖ£ŗAl£ØOH£©3+2OH-=2 AlO2-+H2O£»
£Ø4£©¢ŁæÕ£»
¢Śøł¾ŻŹżÖįæÉÖŖ£¬ČܽāĒāŃõ»ÆĀĮĻūŗĵÄĒāŃõ»ÆÄĘČÜŅŗŹĒ70ml-60ml=10ml£¬ŌņÉś³ÉĒāŃõ»ÆĀĮĻūŗĵÄĒāŃõ»ÆÄĘČÜŅŗĢå»żÓ¦øĆŹĒ30ml£¬ĖłŅŌÉś³ÉĒāŃõ»ÆĆ¾ĻūŗĵÄĒāŃõ»ÆÄĘČÜŅŗĢå»żŹĒ60ml-10ml-30ml=20ml£¬Ōņøł¾Ż·½³ĢŹ½Mg2++2OH-=Mg£ØOH£©2”ż”¢Al3++3OH-=Al£ØOH£©3”żæÉÖŖAlŗĶMgµÄĪļÖŹµÄĮæÖ®±ČŹĒ1£ŗ1µÄ£¬ŌņĀĮµÄÖŹĮæ·ÖŹżŹĒ$\frac{27}{27+24}$”Į100%=52.94%£¬
¹Ź“š°øĪŖ£ŗÄÜ£»52.94%£®
µćĘĄ ±¾Ģāæ¼²éĆ¾ĀĮŗĻ½šÖŠĆ¾ÖŹĮæ·ÖŹż²ā¶ØĢ½¾æŹµŃéµÄÓŠ¹ŲÅŠ¶Ļ£¬ŹĒøßæ¼ÖŠµÄ³£¼ūĢāŠĶ£¬ŹōÓŚÖŠµČÄѶȵďŌĢā£¬ŹŌĢā×ŪŗĻŠŌĒ棬ŌŚ×¢ÖŲ¶Ō»ł“”ÖŖŹ¶¹®¹ĢŗĶѵĮ·µÄĶ¬Ź±£¬²ąÖŲ¶ŌѧɜÄÜĮ¦µÄÅąŃųŗĶ½āĢā·½·ØµÄÖøµ¼ÓėѵĮ·£¬ÓŠĄūÓŚÅąŃųѧɜ¹ę·¶ŃĻ½÷µÄŹµŃéÉč¼ĘÄÜĮ¦ŅŌ¼°ĘĄ¼ŪÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | $\frac{a-b}{V}$ mol•L-1 | B£® | $\frac{2a-b}{V}$ mol•L-1 | C£® | $\frac{2£Øa-b£©}{V}$ mol•L-1 | D£® | $\frac{2£Ø2a-b£©}{V}$ mol•L-1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | æÉ×÷Ź³Ę·µ÷Ī¶¼Į | B£® | æÉ×÷Ź³Ę··ĄøƼĮ | ||
| C£® | æÉ×÷ÅäÖĘ0.9%ÉśĄķŃĪĖ®µÄŌĮĻ | D£® | µē½ā±„ŗĶŹ³ŃĪĖ®æɵƵ½½šŹōÄĘ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ²Ī¼Ó·“Ó¦µÄŃõ»Æ¼ĮÓė»¹Ō¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ1£ŗ5 | |
| B£® | ČōÓŠ2 mol Fe2+±»Ńõ»Æ£¬Ōņ±»Fe2+»¹ŌµÄO2µÄĪļÖŹµÄĮæĪŖ0.5 mol | |
| C£® | ĆæÉś³É1 mol Fe3O4£¬·“Ó¦×ŖŅʵĵē×ÓĪŖ4 mol | |
| D£® | O2ŹĒŃõ»Æ¼Į£¬S2O32-ÓėFe2+ŹĒ»¹Ō¼Į |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 131 | B£® | 53 | C£® | 78 | D£® | 25 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ćŗ | B£® | ÉśĪļÖŹÄÜ | C£® | ĢģČ»Ęų | D£® | ŹÆÓĶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÕÓĘų | B£® | ŹÆÓĶ | C£® | Ćŗ | D£® | ĢģČ»Ęų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĶĖæ | B£® | ČŪČŚµÄMgCl2 | C£® | NaClČÜŅŗ | D£® | ŅŅ“¼ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
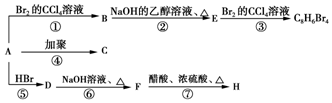
 £®
£® £¬D
£¬D £¬E
£¬E £¬H
£¬H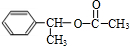 £®
£® +NaOH$”ś_{”÷}^{H_{2}O}$
+NaOH$”ś_{”÷}^{H_{2}O}$ +NaBr£®
+NaBr£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com