
ͭ�����ֳ�����������CuO��Cu2O��ijѧϰС��ȡ0.98g���þ�����ƽ������Cu(OH)2������ȣ���ͭ�����������ɣ����������¶ȱ仯��ͼ1��ʾ�����⣬ijͬѧ������������ʾ����������������������Ԫ�ص������Ĺ�ϵ���ߣ���ͼ2��ʾ�������з�����ȷ���ǣ� ��
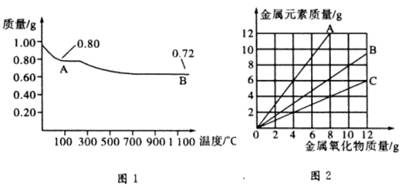
A��ͼ1�У�A��B�Ĺ�������0.01 mol���ӷ�����ת��
B��ͼ1���������й�����0.26 gˮ
C��ͼ2���������У���ʾCuO����������CuԪ��������ϵ��������A
D��ͼ1��A��B��ѧʽ�ֱ�ΪCu2O��CuO
A
����������0.98 g Cu(OH)2��֪�������ʵ���Ϊ0.01 mol����ȫ������CuO��������Ϊ0.01 mol��80 g��mol-1 =0.8g������A����CuO����ȫ������Cu2O��������Ϊ0.005 mol��144 g��mol-1=0.72g������B����Cu2O��D���ȷ�����ݻ�ѧ����ʽ4Cu2O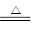 4Cu+2O2����֪����Ӧ�����й�����������������B���ȷ��CuO����������CuԪ�ص�������ϵ����CuO������Ϊ10g���㣩Ϊ��CuO��Cu���۲�ͼ��֪��B��������������ϵ����ʾ����CuO������A���ϵ��κ�һ�㶼��ʾ���������������С��������������Ԫ�ص���������C���ȷ�����Դ�ѡA��
4Cu+2O2����֪����Ӧ�����й�����������������B���ȷ��CuO����������CuԪ�ص�������ϵ����CuO������Ϊ10g���㣩Ϊ��CuO��Cu���۲�ͼ��֪��B��������������ϵ����ʾ����CuO������A���ϵ��κ�һ�㶼��ʾ���������������С��������������Ԫ�ص���������C���ȷ�����Դ�ѡA��


 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ����ʯ���С����ݸ��и���11��������ѧ�Ծ��������棩 ���ͣ�ѡ����
ͭ�����ֳ�����������CuO��Cu2O��ijѧϰС��ȡ0.98 g(�þ�����ƽ����)Cu(OH)2���壬���������ͭ�����������ɣ����������¶ȱ仯��������ͼ1��ʾ��
���⣬ijͬѧ������������ʾ����������������������Ԫ�������Ĺ�ϵ���ߣ���ͼ2��ʾ�������з�����ȷ����
A��ͼ1�в���A��B�Ļ�ѧʽ�ֱ�ΪCu2O��CuO
B��ͼ1���������й�����0.26 g H2O
C��ͼ2���������У���ʾCuO����������CuԪ�������Ĺ�ϵ����������A
D��ͼ2�л��ƴ�������߹�2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ӱ�ʡ������ѧ��һ�����Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
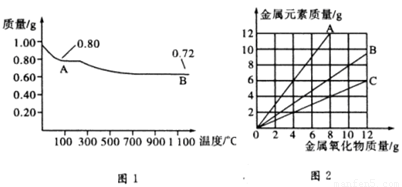
ͭ�����ֳ�����������CuO��Cu2O��ijѧϰС��ȡ0.98g���þ�����ƽ������Cu(OH)2������ȣ���ͭ�����������ɣ����������¶ȱ仯��ͼ1��ʾ�����⣬ijͬѧ������������ʾ����������������������Ԫ�ص������Ĺ�ϵ���ߣ���ͼ2��ʾ�������з�����ȷ����
A��ͼ1�У�A��B�Ĺ�������0.01 mol���ӷ�����ת��
B��ͼ1���������й�����0.26 gˮ
C��ͼ2���������У���ʾCuO����������CuԪ��������ϵ��������A
D��ͼ1��A��B��ѧʽ�ֱ�ΪCu2O��CuO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ������һ��ģ���������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
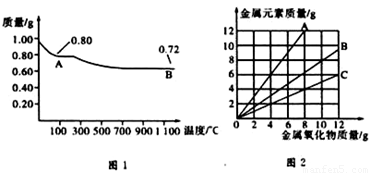
ͭ�����ֳ����������CuO��Cu2O��ijѧϰС��ȡ0.98gCu(OH)2������ȣ���ͭ�����������ɣ����������¶ȱ仯��ͼ1��ʾ�����⣬ijͬѧ������������ʾ����������������������Ԫ�ص������Ĺ�ϵ���ߣ���ͼ2��ʾ��

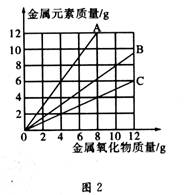
�����з�����ȷ���ǣ� ��
A��ͼ1�в���A��B�Ļ�ѧʽ�ֱ�ΪCu2O��CuO
B��ͼ1���������й�����0.26gˮ
C��ͼ2���������У���ʾCuO����������CuԪ��������ϵ��������C
D��ͼ1�У�A��B��������0.01mol���ӷ�����ת��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com