
ФГpHЃН1ЕФЙЄвЕЗЯвКЃЌжЛПЩФмКЌгавдЯТРызгжаЕФШєИЩжжЃКHЃЋЁЂMg2ЃЋЁЂBa2ЃЋЁЂClЃЁЂCO32-ЁЂSO42-ЃЌЯжШЁСНЗн100 mLШмвКНјааШчЯТЪЕбщЃК
ЕквЛЗнМгШызуСПAgNO3ШмвКЃЌЕУИЩдяГСЕэ3.50 gЁЃ
ЕкЖўЗнМгзуСПBaCl2ШмвККѓЃЌЕУИЩдяГСЕэ2.33 gЃЌОзуСПбЮЫсЯДЕгЁЂИЩдяКѓЃЌГСЕэжЪСПВЛБфЁЃ
ИљОнЩЯЪіЪЕбщЃЌвдЯТЭЦВте§ШЗЕФЪЧЃЈ ЃЉ
ЂйвЛЖЈДцдкMg2ЃЋЁЁЂкПЩФмДцдкCO32-ЁЁЂлвЛЖЈДцдкClЃЁЁЂмПЩФмДцдкBa2ЃЋЁЁЂнПЩФмДцдкMg2ЃЋ
AЃЎЂйЂл BЃЎЂкЂл CЃЎЂлЂн DЃЎЂмЂн

| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ИпПМЛЏбЇЖўТжзЈЬтЭЛЦЦ зЈЬтЪЎЖўГЃМћЗЧН№ЪєдЊЫиСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
вбжЊAЁЂBЁЂCЁЂDЪЧжабЇЛЏбЇЕФГЃМћЮяжЪЃЌЧвAЁЂBЁЂCОљКЌгаЭЌвЛжждЊЫиЁЃдквЛЖЈЬѕМўЯТЫќУЧжЎМфЕФЯрЛЅзЊЛЏЙиЯЕШчЭМЫљЪО(ВПЗжЗДгІжаЕФH2OвбТдШЅ)ЁЃ
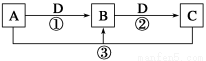
ЧыЛиД№ЯТСаЮЪЬтЃК
(1)ШєAПЩгУгкздРДЫЎЯћЖОЃЌDЪЧЩњВњЁЂЩњЛюжагУСПзюДѓЁЂгУЭОзюЙуЕФН№ЪєЕЅжЪЃЌМгШШеєИЩBЕФШмвКВЛФмЕУЕНBЃЌдђBЕФЛЏбЇЪНПЩФмЪЧ____________________ЃЛЙЄвЕЩЯжЦШЁAЕФРызгЗНГЬЪНЮЊ______________________ЁЃ
(2)ШєAЪЧвЛжжМюадЦјЬхЃЌГЃгУзїжЦРфМСЃЌBЪЧЦћГЕЮВЦјжЎвЛЃЌгіПеЦјЛсБфЩЋЃЌдђЗДгІЂйЕФЛЏбЇЗНГЬЪНЮЊ________________________ЁЃ
(3)ШєDЪЧТШМюЙЄвЕЕФжївЊВњЦЗжЎвЛЃЌBгаСНадЃЌдђЗДгІЂкЕФРызгЗНГЬЪНЪЧ____________________ЁЃ
(4)ШєAЁЂCЁЂDЖМЪЧГЃМћЦјЬхЃЌCЪЧЕМжТЫсгъЕФжївЊЦјЬхЃЌдђЗДгІЂлЕФЛЏбЇЗНГЬЪНЮЊ____________________ЁЃ
ФГЭЌбЇНЋЫбМЏЕНЕФвЛЖЈСПЕФЫсгъБЃДцдкУмБеШнЦїжаЃЌУПИєвЛЖЈЪБМфВтЫсгъЕФpHЃЌЗЂЯждкЦ№ЪМвЛЖЮЪБМфФкЃЌЫсгъЕФpHГЪМѕаЁЧїЪЦЃЌгУРызгЗНГЬЪННтЪЭдвђЃК_______________
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ИпПМЛЏбЇЖўТжзЈЬтЭЛЦЦ зЈЬтСљЮяжЪНсЙЙКЭдЊЫижмЦкТЩСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
XЁЂYЁЂZЁЂQЁЂRЪЧЮхжжЖЬжмЦкдЊЫиЃЌдзгађЪ§вРДЮдіДѓЁЃXЁЂYСНдЊЫизюИпе§МлгызюЕЭИКМлжЎКЭОљЮЊ0ЃЛQгыXЭЌжїзхЃЛZЁЂRЗжБ№ЪЧЕиПЧжаКЌСПзюИпЕФЗЧН№ЪєдЊЫиКЭН№ЪєдЊЫиЁЃ
ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЮхжждЊЫидзгАыОЖгЩДѓЕНаЁЕФЫГађЪЧ(аДдЊЫиЗћКХ)____________________ЁЃ
ЃЈ2ЃЉXгыYФмаЮГЩЖржжЛЏКЯЮяЃЌЦфжаМШКЌМЋадМќгжКЌЗЧМЋадМќЃЌЧвЯрЖдЗжзгжЪСПзюаЁЕФЮяжЪЪЧ(аДЗжзгЪН)________________ЁЃ
ЃЈ3ЃЉгЩвдЩЯФГаЉдЊЫизщГЩЕФЛЏКЯЮяAЁЂBЁЂCЁЂDгаШчЯТзЊЛЏЙиЯЕЃКA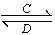 B(дкЫЎШмвКжаНјаа)
B(дкЫЎШмвКжаНјаа)
ЦфжаЃЌCЪЧШмгкЫЎЯдЫсадЕФЦјЬхЃЛDЪЧЕЛЦЩЋЙЬЬхЁЃ
аДГіCЕФНсЙЙЪНЃК________ЃЛDЕФЕчзгЪНЃК________ЁЃ
ЂйШчЙћAЁЂBОљгЩШ§жждЊЫизщГЩЃЌBЮЊСНадВЛШмЮяЃЌдђAЕФЛЏбЇЪНЮЊ________________________ЃЛгЩAзЊЛЏЮЊBЕФРызгЗНГЬЪНЮЊ___________________ЁЃ
ЂкШчЙћAгЩШ§жждЊЫизщГЩЃЌBгЩЫФжждЊЫизщГЩЃЌAЁЂBШмвКОљЯдМюадЁЃгУРызгЗНГЬЪНБэЪОAШмвКЯдМюадЕФдвђЃК
________________________________________________________________________ЁЃ
AЁЂBХЈЖШОљЮЊ0.1 molЁЄLЃ1ЕФЛьКЯШмвКжаЃЌРызгХЈЖШгЩДѓЕНаЁЕФЫГађЪЧ____________________ЃЛГЃЮТЯТЃЌдкИУШмвКжаЕЮМгЯЁбЮЫсжСжаадЪБЃЌШмжЪЕФжївЊГЩЗжга________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ИпПМЛЏбЇЖўТжзЈЬтЭЛЦЦ зЈЬтАЫЕчЛЏбЇСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯрЭЌВФжЪЕФЬњдкЭМжаЫФжжЧщПіЯТзюВЛвзБЛИЏЪДЕФЪЧЃЈ ЃЉ

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ИпПМЛЏбЇЖўТжзЈЬтЭЛЦЦ зЈЬтАЫЕчЛЏбЇСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ФГаЁзщЮЊбаОПЕчЛЏбЇдРэЃЌЩшМЦШчЭМзАжУЁЃЯТСаа№Ъіе§ШЗЕФЪЧЃЈ ЃЉ
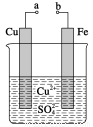
AЃЎaКЭbгУЕМЯпСЌНгЪБЃЌЬњЦЌЩЯЛсгаН№ЪєЭЮіГі
BЃЎaКЭbгУЕМЯпСЌНгЪБЃЌЭЦЌЩЯЗЂЩњЕФЗДгІЮЊ2HЃЋЃЋ2eЃ=H2Ёќ
CЃЎЮоТлaКЭbЪЧЗёСЌНгЃЌЬњЦЌОљЛсШмНтЃЌШмвКДгРЖЩЋж№НЅБфГЩЧГТЬЩЋ
DЃЎaКЭbЗжБ№СЌНгжБСїЕчдДе§ЁЂИКМЋЃЌЕчбЙзуЙЛДѓЪБЃЌCu2ЃЋЯђЭЕчМЋ
вЦЖЏ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ИпПМЛЏбЇЖўТжзЈЬтЭЛЦЦ зЈЬтЮхРызгЗДгІСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТСагаЙиЕФРызгЗНГЬЪНе§ШЗЕФЪЧЃЈ ЃЉ
AЃЎТШЛЏТСШмвКжаМгШыЙ§СПАБЫЎЃКAl3ЃЋЃЋ4NH3ЁЄH2O=AlO2-ЃЋ4NH4+
BЃЎЭЦЌНгЕчдДе§МЋЃЌЬМАєНгЕчдДИКМЋЃЌЕчНтСђЫсШмвКЃКCuЃЋ2HЃЋ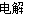 Cu2ЃЋЃЋH2Ёќ
Cu2ЃЋЃЋH2Ёќ
CЃЎСзЫсвЛЧтФЦШмвКЫЎЁОНтЮіЁП
HPO42-ЃЋH2O PO43-ЃЋH3OЃЋ
PO43-ЃЋH3OЃЋ
DЃЎЪЕбщЪвХфжЦЕФбЧЬњбЮШмвКдкПеЦјжаБЛбѕЛЏЃК4Fe2ЃЋЃЋO2ЃЋ2H2O=4Fe3ЃЋЃЋ4OHЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ИпПМЛЏбЇЖўТжзЈЬтЭЛЦЦ зЈЬтЖўЛЏбЇгУгяМАГЃгУМЦСПСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЃЈ1ЃЉдквЛЖЈЮТЖШКЭбЙЧПЯТЃЌ1ЬхЛ§X2ЃЈgЃЉКЭ3ЬхЛ§Y2ЃЈgЃЉЛЏКЯЩњГЩ2ЬхЛ§ZЃЈgЃЉЃЌдђZЦјЬхЕФЛЏбЇЪНЪЧ ЁЃ
ЃЈ2ЃЉAЁЂBСНжжЦјЬхзщГЩЕФЛьКЯЦјЬх8.6 gЃЌдкБъзМзДПіЯТЬхЛ§ЮЊ8.96 LЁЃвбжЊAгыBЕФЮяжЪЕФСПжЎБШЮЊ3ЁУ1ЃЌЯрЖдЗжзгжЪСПжЎБШЮЊ14ЁУ1ЃЌгЩДЫПЩЭЦЖЯAПЩФмЪЧ ЃЌBПЩФмЪЧ ЁЃ
ЃЈ3ЃЉдкБъзМзДПіЯТЃЌCOКЭCO2ЕФЛьКЯЦјЬхжЪСПЮЊ36 gЃЌЬхЛ§ЮЊ22.4 LЃЌдђCOЫљеМЕФЬхЛ§ЪЧ LЃЌжЪСПЪЧ gЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ИпПМЛЏбЇЖўТжзЈЬтЭЛЦЦ зЈЬтОХЛЏбЇЗДгІЫйТЪЛЏбЇЦНКтСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
вЛЖЈЮТЖШЪБЃЌЯђ2.0 LКуШнУмБеШнЦїжаГфШы2 mol SO2КЭ1 mol O2ЃЌЗЂЩњЗДгІЃК
2SO2(g)ЃЋO2(g)  2SO3(g)ЁЃОЙ§вЛЖЮЪБМфКѓДяЕНЦНКтЁЃЗДгІЙ§ГЬжаВтЖЈЕФВПЗжЪ§ОнМћЯТБэЃК
2SO3(g)ЁЃОЙ§вЛЖЮЪБМфКѓДяЕНЦНКтЁЃЗДгІЙ§ГЬжаВтЖЈЕФВПЗжЪ§ОнМћЯТБэЃК
t/s | 0 | t1 | t2 | t3 | t4 |
n(SO3)/mol | 0 | 0.8 | 1.4 | 1.8 | 1.8 |
ЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
AЃЎЗДгІдкЧАt1sЕФЦНОљЫйТЪv(O2)ЃН0.4/t1 molЁЄLЃ1ЁЄsЃ1
BЃЎБЃГжЦфЫћЬѕМўВЛБфЃЌЬхЛ§бЙЫѕЕН1.0 LЃЌЦНКтГЃЪ§НЋдіДѓ
CЃЎЯрЭЌЮТЖШЯТЃЌЦ№ЪМЪБЯђШнЦїжаГфШы4 mol SO3ЃЌДяЕНЦНКтЪБЃЌSO3ЕФзЊЛЏТЪДѓгк10%
DЃЎБЃГжЮТЖШВЛБфЃЌЯђИУШнЦїжадйГфШы2 mol SO2ЁЂ1 mol O2ЃЌЗДгІДяЕНаТЦНКтЪБn(SO3)/n(O2)діДѓ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ИпПМЛЏбЇЖўТжзЈЬтЭЛЦЦ зЈЬтвЛЮяжЪЕФзщГЩаджЪКЭЗжРрСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ФГКЯзїбЇЯАаЁзщЬжТлБцЮівдЯТЫЕЗЈЃЌЦфжае§ШЗЕФЪЧЃЈ ЃЉ
ЂйДжбЮКЭЫсгъЖМЪЧЛьКЯЮя
ЂкегЦјКЭЫЎУКЦјЖМЪЧПЩдйЩњФмдД
ЂлБљКЭИЩБљМШЪЧДПОЛЮягжЪЧЛЏКЯЮя
ЂмВЛатИжКЭФПЧАСїЭЈЕФгВБвЖМЪЧКЯН№
ЂнДПМюКЭЪьЪЏЛвЖМЪЧМю
ЂоЖЙНЌКЭЮэЖМЪЧНКЬх
AЃЎЂйЂкЂлЂм BЃЎЂйЂкЂнЂо
CЃЎЂлЂнЂо DЃЎЂйЂлЂмЂо
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com