
������Ԫ��X��Y��Z��W ��ԭ����������������ԭ������������֮��Ϊ13��X ��
ԭ�Ӱ뾶��Y ��С��X ��W ͬ���壬Z �ǵؿ��к�����ߵ�Ԫ�ء�����˵����ȷ���ǣ� ��
A��ԭ�Ӱ뾶�Ĵ�С˳��: r(Y)>r(Z)>r(W)
B��Ԫ��Z��W �ļ����ӵĵ��Ӳ�ṹ��ͬ
C��Ԫ��Y �ļ���̬�⻯������ȶ��Ա�Z ��ǿ
D��ֻ��X��Y��Z ����Ԫ�صĻ�������������ӻ����Ҳ�����ǹ��ۻ�����
 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ4���廷��ϩ��������4����ͬ��Ӧ�����У�����ֻ
����һ�ֹ����ŵķ�Ӧ��
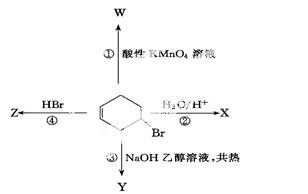
A���٢ܡ����� B���ڢ� C���٢ڡ����� D���ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ�����ڱ��еڢ�A��Ԫ�صĵ��ʼ��仯�������;�㷺��
(1)����Ԫ��ͬ��Ķ�����Ԫ�ص�ԭ�ӽṹʾ��ͼΪ_________��
(2)����Ϊ�ȡ��塢��Ԫ�طǽ�����(ԭ�ӵõ�������)�ݱ���ɵ��ж�������________(��ѡ����ĸ)��
a��Cl2��Br2��I2���۵�
b��Cl2��Br2��I2��������
c��HCl��HBr��HI�����ȶ���
d��HCl��HBr��HI������
(3)��ҵ�ϣ�ͨ������ת�����Ƶ�KClO3���壺
NaCl��Һ NaClO3��Һ
NaClO3��Һ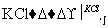 KClO3����
KClO3����
����ɢ��з�Ӧ���ܻ�ѧ����ʽ�� NaCl��
NaCl�� H2O===
H2O=== NaClO3��
NaClO3�� ________��
________��
�ڢ���ת���Ļ�����Ӧ������__________���÷�Ӧ����������KClO3���������������������ԭ����_________________��
(4)һ�������£���ˮ��Һ��1 mol Cl����ClO (x��1,2,3,4)������(kJ)��Դ�С��ͼ��ʾ��
(x��1,2,3,4)������(kJ)��Դ�С��ͼ��ʾ��

��D��________(�����ӷ���)��
��B�D��A��C��Ӧ���Ȼ�ѧ����ʽΪ_______________________(�����ӷ��ű�ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�Ԫ�����ʵ�˵������ȷ���� (����)
A���������е����Ų�ʽ��ԭ���У���1s22s22p63s23p2
��1s22s22p3����1s22s22p2����1s22s22p63s23p4��ԭ�Ӱ뾶�����Ǣ�
B�������������������Ų�ʽ��ԭ���У���3s23p1����3s23p2����3s23p3����3s23p4��һ�����������Ǣ�
C����Na��K��Rb����N��P��As����O��S��Se����Na��P��Cl��Ԫ�صĵ縺����ԭ������������������Ǣ�
D��ijԪ����̬��̬ԭ�ӵ������ֱܷ�Ϊ738��1 451��7 733��10 540��13 630��17 995��21 703��������������Ӧʱ�������ɵ���������X3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й��������ʵ�˵���������
A�����ȶ��ԣ�HCl> HI B��ԭ�Ӱ뾶��Na> Mg
C�����ԣ�H2SO3>H2SO4�� D���������������S2-> Cl-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��ΪԪ�����ڱ���ǰ20��Ԫ�أ�������ӵĵ��Ӳ�ṹ��ͬ������˵����ȷ����
A��X2���Ļ�ԭ��һ������Y��
B����mXa����nYb����m��a��n��b
C��X��Yһ������ͬ����Ԫ��
D����X��ԭ�Ӱ뾶����Y������̬�⻯����ȶ���һ����X����Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�������£���ȫ�ֽ�����ij������2 g����������1.6 g���˻�����������
A��1H216O B��2H216O C��1H218O D��1H218O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪A��B��C��D��Ϊ������Ԫ�أ����ǵ�ԭ��������������A��ԭ���������һ�����ӵķǽ���Ԫ
�أ�Cԭ�ӵ������������Ǵ�����������3����C��D���γ����ֹ�̬���������һ��Ϊ����ɫ��
�壻B��C���γɶ�����̬�����A��B��C����Ԫ�ؿ��γ����ӻ�����û������и�Ԫ��ԭ�ӵ�����
����֮��Ϊn(A)��n(B)��n(C)��4��2��3����ش��������⣺
��1��д��C��D�γɵĵ���ɫ���廯����ĵ���ʽ��____________________________��
��2����Ԫ��ԭ�ӵ����ʵ���֮��Ϊn(A)��n(B)��n(C)��4��2��3�Ļ����������Ϊ________����ˮ��Һ��________�ԡ�
��3����д����A2C��BA3�����е�������ͬ��������A��B��CԪ������������Ԫ����ɵ����ӵķ���(������)_________________________��________��
��4��д����B��CԪ����ɵ�Ԫ��ԭ��������Ϊn(B)��n(C)��7��12�Ļ�����Ļ�ѧʽ________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣������������ʣ���HNO3���ڱ����ᡡ�۰�ˮ����Al(OH)3����NaHCO3(s)����Cu������ˮ����CaCO3 ��H2CO3 ������
��1����������������ǿ����ʵ���________������������ʵ���________��
��2��д���������ʵĵ��뷽��ʽ��
�ڡ�______________________���ܡ�______________________��
�ݡ�______________________���ᡢ ��
��4�֣���ѧ��Ӧ4A(s)��3B(g)  2C(g)��D(g)��10 minC��Ũ������0.6 mol/L��
2C(g)��D(g)��10 minC��Ũ������0.6 mol/L��
��1����B��ʾ�ķ�Ӧ������________ ��
��2���ֱ���B��C��D��ʾ�ķ�Ӧ�������ֵ��________ ��
��8�֣�����������⣺
��1�����͵���Ҫ�ɷ������飨C8H18����1molC8H18��l������O2��g����ȼ�գ�����CO2��g����H2O��l�����ų�5518KJ�������÷�Ӧ�Ȼ�ѧ����ʽΪ��
��
��2����֪���ʯ��ʯī�ֱ�����������ȫȼ�յ��Ȼ�ѧ����ʽΪ��
C�����ʯ��s)��O2(g)��CO2(g) ��H����395.41kJ/mol
C��ʯī��s����O2(g)��CO2(g) ��H����393.51kJ/mol
��ʯīת��Ϊ���ʯ���Ȼ�ѧ����ʽΪ��________ ���ɴ˿������ʯ���ȶ��� ������ڡ�����С�ڡ�������ȷ������ʯī���ȶ��ԡ�
��3������������N2H4Ϊȼ�ϣ�NO2Ϊ�����������߷�Ӧ����֪��
N2(g) ��2O2(g) ��2NO2(g) �SH��67.7kJ/mol
N2H4(g) ��O2(g) �� N2(g) ��2H2O(g) �SH����534kJ/mol
H2 O (l) ��H2O (g) ��H��44 kJ/mol
1molN2H4(g)�� NO2��g����ȫ��Ӧ����N2��H2 O (l)ʱ�ų�����Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com