
�ⶨNa2S2O3·5H2O��Ʒ����
ȷ��ȡW g Na2S2O3·5H2O��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.100 0 mol·L��1��ı���Һ�ζ���
��Ӧԭ��Ϊ2S2O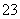 ��I2===S4O
��I2===S4O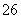 ��2I��
��2I��

(5)�ζ����յ�ʱ����Һ��ɫ�ı仯��___________________________��
(6)�ⶨ��ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ________mL����Ʒ�Ĵ���Ϊ(��Na2S2O3·5H2O��Է�������ΪM)________��
�𰸡�(5)����ɫ����ɫ
(6)18.10��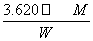 ��100%
��100%
������(5)�õ�����ָʾ�����ﵽ�յ�ʱ����������Һ����ɫ��Ϊ��ɫ��
(6)����ͼʾ���ζ��ܵĶ�����Ҫ����2λС�����������ĵ�ı���Һ�����Ϊ18.10 mL��
����2S2O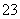 ��I2===S4O
��I2===S4O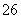 ��2I��֪��n(S2O
��2I��֪��n(S2O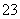 )��2n(I2)��0.100 0 mol·L��1��18.10 mL��10��3 L·mL��1��2��3.620��10��3 mol����Ʒ�Ĵ���Ϊ3.620��10��3 mol��M/W��100%��
)��2n(I2)��0.100 0 mol·L��1��18.10 mL��10��3 L·mL��1��2��3.620��10��3 mol����Ʒ�Ĵ���Ϊ3.620��10��3 mol��M/W��100%��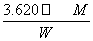 ��100%��
��100%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͬѧ��Ϊ����ˮ�м���H2SO4��ˮ�ĵ���ƽ�������ƶ��������Ǽ���H2SO4��c(H��)����ƽ�����ơ���ͬѧ��Ϊ������H2SO4��ˮ�ĵ���ƽ�������ƶ�������Ϊ����H2SO4��c(H��)Ũ������H����OH���кͣ�ƽ�����ơ�����Ϊ����˵����ȷ����˵��ԭ��ˮ�ĵ���ƽ���ƶ�����Һ��c(H��)·c(OH��)�������Ǽ�С��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
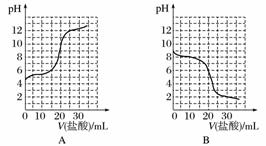
��֪ij�¶���CH3COOH�ĵ��볣��K��1.6��10��5�����¶��£���20 mL 0.01 mol·L��1 CH3COOH��Һ����μ���0.01 mol·L��1 KOH��Һ����pH�仯������ͼ��ʾ(�����¶ȱ仯)����ش������й����⣺
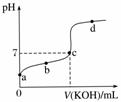
(1)a����Һ��c(H��)Ϊ________��pHԼΪ________��
(2)a��b��c��d�ĵ���ˮ�ĵ���̶�������________���ζ���������ѡ��__________��ָʾ�����ζ��յ���________(�c�����ϡ���c�����¡�)��
(3)����20 mLϡ��ˮ����μ����Ũ�ȵ����ᣬ�����б仯������ȷ����________(����ĸ)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾˮ��c(H��)��c(OH��)�Ĺ�ϵ�������жϴ������ (����)

A������������������c(H��)��c(OH��)��Kw
B��M��������������c(H��)��c(OH��)
C��ͼ��T1��T2
D��XZ������������pH��7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
0.1 mol·L��1ij��AOH��Һ��pH��11��������Һϡ��10������Һ��pH������Ϊ(����)
��10.1 ��10.8 ��12 ��11.5
A���ۢ� B���٢�
C���٢� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijУ��ѧ��ȤС���ͬѧ��һ��������Na2SO4��NaOH��Ʒ��NaOH�ĺ������вⶨ����ش��������⣺
(1)��ͬѧ���ó������ⶨ��Ʒ��NaOH�ĺ�������ͬѧѡ�õ�ҩƷ����Ʒ�⣬��Ӧ��________��ʵ����Ӧ�ⶨ��������________��
(2)��ͬѧ���õζ����ⶨ��Ʒ��NaOH�ĺ�����
���÷�����ƽȷ��ȡ����Ʒ5.000 0 g��ȫ������ˮ���Ƴ�1 000.0 mL��Һ���ü�ʽ�ζ�����ȡ20.00 mL������Һ������ƿ�У��μӼ���ָʾ�������⡣�ζ�����ʹ��ǰ��ϴ���⣬��Ӧ____________________________________��
����Ũ��Ϊ0.100 0 mol·L��1���������Һ���еζ�����ʼ�ζ�ǰ��һ��������________��
�۵ζ���������pH�Ʋⶨ��ƿ����Һ��pH���ٽ��ζ��յ�ʱ�ⶨpHӦÿ��һ�β�һ�Ρ�
�ܵζ������У���ƿ����Һ��pH�仯���£�
| V(HCl)/mL | 0.00 | 12.00 | 18.00 | 22.00 | 23.00 | 23.96 | 24.00 | 24.04 | 25.00 | 26.00 | 30.00 |
| pH | 13.1 | 12.6 | 12.2 | 11.7 | 11.4 | 9.9 | 7.0 | 4.0 | 2.7 | 2.4 | 1.9 |
��������ͼ�л��Ƴ������к͵ζ������ߡ�
�������ʾ�Ǽ������ָʾ���ı�ɫ��Χ���������������к͵ζ����߷����������к͵ζ���Ӧѡ�õ�ָʾ����________��
| ָʾ�� | ��ɫ��Χ(pH) | ��ɫ | |
| �� | �� | ||
| ���� | 3.1��4.4 | �� | �� |
| ʯ�� | 5.0��8.0 | �� | �� |
| ��̪ | 8.2��10.0 | �� | �� |
����Ʒ�У�NaOH�������ٷֺ���Ϊ____________��
�𰸡�(1)MgCl2��Һ����Ʒ�����ͼ�������MgCl2��Һ�����ɵij�������
(2)�ټ�©����ϴ���ڵ���ʢ��Һ�ĵζ��ܵ�Һ���ڡ�0���̶Ȼ�0���̶�����
�ܵζ�������ͼ��ʾ
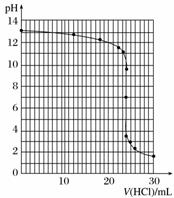
�ݼ��Ȼ��̪����96%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ϣ�ش��������⣺
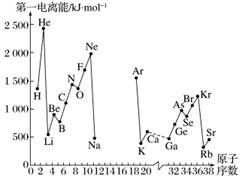
A.��һ������I1��ָ��̬ԭ��X(g)���ڻ�̬ʱ��ʧȥһ�����ӳ�Ϊ��̬������X��(g)����������������ͼ�Dz���Ԫ��ԭ�ӵĵ�һ������I1��ԭ�������仯������ͼ(����12����17��Ԫ�ص��й�����ȱʧ)��
B.��ͬԪ�ص�ԭ���ڷ������������ӵ�������С������ֵ��ʾ������ֵ��Ϊ�縺�ԡ�һ����Ϊ����������ɼ�ԭ�Ӽ�ĵ縺�Բ�ֵ����1.7��ԭ��֮��ͨ���γ����Ӽ�����������ɼ�ԭ�Ӽ�ĵ縺�Բ�ֵС��1.7��ͨ���γɹ��ۼ����±���ijЩԪ�صĵ縺��ֵ��
| Ԫ�ط��� | Li | Be | B | C | O | F | Na | Al | Si | P | S | Cl |
| �縺��ֵ | 1.0 | 1.5 | 2.0 | 2.5 | 3.5 | 4.0 | 0.9 | 1.5 | 1.8 | 2.1 | 2.5 | 3.0 |
(1)���������ϢAͼ��ͬ����Ԫ�ص�һ�����ܵı仯���ɣ��ƶϵ�������Na��Ar�⼸��Ԫ���У�Al�ĵ�һ�����ܵĴ�С��ΧΪ______��Al��______(��Ԫ�ط���)��
(2)����ϢAͼ�з�����֪��ͬһ����Ԫ��ԭ�ӵĵ�һ������I1�ı仯������________________��
(3)��ϢAͼ�е�һ��������С��Ԫ�������ڱ��е�λ����________����________�塣
(4)���ݶԽ��߹���Be��AlԪ������������Ӧˮ������������ƣ����Ƕ�����________�ԣ�����Be(OH)2��ʾ�������ʵ����ӷ���ʽ��________________________________________________________________________��
(5)ͨ�������縺��ֵ�ı仯���ɣ�ȷ��MgԪ�صĵ縺��ֵ����С��Χ________________��
(6)�����Ԫ�صĵ縺�Ժͽ����ԡ��ǽ����ԵĹ�ϵ��________________��
(7)�ӵ縺�ԽǶȣ��ж�AlCl3�����ӻ����ﻹ�ǹ��ۻ����˵�����ɲ�д���жϵķ���________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڻ�ѧ����˵����ȷ����(����)
A�����ɵ��ʷ��ӵ�������һ�����й��ۼ�
B���ɷǽ���Ԫ����ɵĻ����ﲻһ���ǹ��ۻ�����
C���Ǽ��Լ�ֻ������˫ԭ�ӵ��ʷ�����
D����ͬԪ����ɵĶ�ԭ�ӷ����еĻ�ѧ��һ���Ǽ��Լ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com