
ŅŃÖŖijŅ»ÖÖČ¼ĮĻŗ¬Ģ¼”¢ĒāŗĶŃõČżÖÖŌŖĖŲ£¬ĪŖ²ā¶ØøĆČ¼ĮĻµÄ×é³É£ŗ½«øĆČ¼ĮĻ·ÅČėµ½×ćĮæŃõĘųÖŠČ¼ÉÕ£¬²¢Ź¹²śÉśµÄCO2ÓėH2OÕōĘųŅŌ¼°Ź£ÓąµÄO2Č«²æĶعżČēĻĀĶ¼ĖłŹ¾µÄ×°ÖĆ£¬µĆµ½ČēĻĀ±ķĖłĮŠ³öµÄŹµŃ鏿¾Ż£Ø¼ŁÉčÉś³ÉµÄĘųĢåŹĒČ«²æ±»ĪüŹÕ£©”£
| | ŹµŃéĒ° | ŹµŃéŗó |
| ¼×µÄÖŹĮæ / g | 101£®1 | 103£®8 |
| ŅŅµÄÖŹĮæ / g | 82£®0 | 86£®4 |
1:3£»C2H6O
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©·ÖĪöĢāøų×°ÖĆÖŖ£¬ÅØĮņĖįÓĆĄ“ĪüŹÕĖ®ÕōĘų£¬¼××°ÖĆŌö¼ÓµÄÖŹĮæĪŖH2OµÄÖŹĮ棬m(H2O)=103£®8g-101£®1g=2£®7g£¬n(H2O)=0.15mol£¬n(H)=0.3mol£»¼īŹÆ»ŅÓĆĄ“ĪüŹÕCO2£¬ŅŅ×°ÖĆŌö¼ÓµÄÖŹĮæĪŖCO2µÄÖŹĮ棬m(CO2)=86£®4g-82£®0g=4£®4g£¬n(C)=n(CO2)=0.1mol£¬Ģ¼”¢ĒāŌ×ӵďżÄæ±ČĪŖ1:3”££Ø2£©ÉčøĆ·Ö×Óŗ¬ÓŠnøöĢ¼Ō×Ó£¬ŌņĒāŌ×ÓĪŖ3nøö£¬Ļą¶Ō·Ö×ÓÖŹĮæĪŖ12n+3n+16=46£¬¼“n=2£¬øĆ·Ö×ÓÓŠ2øöĢ¼Ō×Ó£¬6øöĒāŌ×ÓŗĶŅ»øöŃõŌ×Ó£¬¹ŹøĆĪļÖŹµÄ·Ö×ÓŹ½ĪŖC2H6O”£
æ¼µć£ŗæ¼²éÓŠ»śĪļ·Ö×ÓŹ½µÄČ·¶Ø”£


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
Ļć²ŻČ©ŹĒŅ»ÖÖŹ³Ę·Ģķ¼Ó¼Į£¬æÉÓÉÓś““ľ·Ó×÷ŌĮĻŗĻ³É£¬ŗĻ³ÉĀ·ĻßČēĻĀĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £© 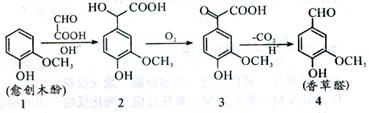
| A£®ĄķĀŪÉĻ·“Ó¦1”ś2ÖŠŌ×ÓĄūÓĆĀŹĪŖ100% |
| B£®»ÆŗĻĪļ2²»ÄÜ·¢ÉśĖõ¾Ū·“Ó¦ |
| C£®¼ģŃéÖʵƵÄĻć²ŻČ©ÖŠŹĒ·ń»ģÓŠ»ÆŗĻĪļ3£¬æÉÓĆĀČ»ÆĢśČÜŅŗ |
| D£®µČĪļÖŹµÄĮæµÄĖÄÖÖ»ÆŗĻĪļ·Ö±šÓė×ćĮæNaOH·“Ó¦£¬ĻūŗÄNaOHĪļÖŹµÄĮæÖ®±ČĪŖ1£ŗ3£ŗ2£ŗ4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø8·Ö£©Čā¹šĖį¼×õ„ŹĒ³£ÓĆÓŚµ÷ÖĘ¾ßÓŠ²ŻŻ®”¢ĘĻĢŃ”¢Ó£ĢŅ”¢Ļć×ÓĄ¼µČĻćĪ¶µÄŹ³ÓĆĻć¾«”£ĖüµÄ·Ö×ÓŹ½ĪŖC10H10O2£¬ĒŅ·Ö×ÓÖŠÖ»ŗ¬ÓŠ1øö±½»·£¬±½»·ÉĻÖ»ÓŠŅ»øöČ”“ś»ł”£ŅŃÖŖijŅ»ÖÖČā¹šĖį¼×õ„µÄ·Ö×Ó½į¹¹Ä£ŠĶČēĻĀĶ¼ĖłŹ¾£ØĶ¼ÖŠĒņÓėĒņÖ®¼äĮ¬Ļß±ķŹ¾µ„¼ü»ņĖ«¼ü£©”£
ŹŌ»Ų“šĻĀĮŠĪŹĢā”£
Čā¹šĖį¼×õ„µÄ½į¹¹¼ņŹ½ĪŖ ”££Ø2£©·Ö×ÓÖŠµÄ¹ŁÄÜĶŵÄĆū³ĘĪŖ ”£
£Ø3£©ŌŚ±½»·ÉĻµÄŅ»ĀČ“śĪļÓŠ ÖÖ”£
£Ø4£©Š“³öøĆČā¹šĖį¼×õ„ŌŚĒāŃõ»ÆÄĘČÜŅŗÖŠĖ®½āµÄ»Æѧ·½³ĢŹ½£ŗ_________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©Ķ黳Ȕ“ś±½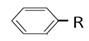 æÉŅŌ±»KMnO4ĖįŠŌČÜŅŗŃõ»Æ³É
æÉŅŌ±»KMnO4ĖįŠŌČÜŅŗŃõ»Æ³É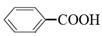 £¬µ«ČōĶ黳RÖŠÖ±½ÓÓė±½»·ĻąĮ¬µÄĢ¼Ō×ÓÉĻƻӊC”ŖH¼ü£¬Ōņ²»Ņ×±»Ńõ»ÆµĆµ½
£¬µ«ČōĶ黳RÖŠÖ±½ÓÓė±½»·ĻąĮ¬µÄĢ¼Ō×ÓÉĻƻӊC”ŖH¼ü£¬Ōņ²»Ņ×±»Ńõ»ÆµĆµ½ ”£ĻÖÓŠ·Ö×ÓŹ½ŹĒC11H16µÄŅ»Ķ黳Ȕ“ś±½£¬ŅŃÖŖĖüæÉŅŌ±»Ńõ»Æ³É
”£ĻÖÓŠ·Ö×ÓŹ½ŹĒC11H16µÄŅ»Ķ黳Ȕ“ś±½£¬ŅŃÖŖĖüæÉŅŌ±»Ńõ»Æ³É µÄŅģ¹¹Ģå¹²ÓŠ7ÖÖ£¬ĘäÖŠĮ½ÖÖŹĒ
µÄŅģ¹¹Ģå¹²ÓŠ7ÖÖ£¬ĘäÖŠĮ½ÖÖŹĒ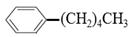 ”¢
Ӣ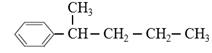 ӣ
ӣ
ĒėŠ“³öĘäĖū5ÖֵĽį¹¹¼ņŹ½£ŗ_____________£»______________£»__________________£»_____________£»____________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø16·Ö£©
£ØI£©ĻĀŹö·“Ó¦ÖŠ£¬ŹōÓŚŃõ»Æ·“Ó¦µÄŹĒ_______ŹōÓŚČ”“ś·“Ó¦µÄŹĒ_______ŹōÓŚ¼Ó³É·“Ó¦µÄŹĒ_______£ØĢīŠņŗÅ£©
¢ŁŅŅ“¼ÓėĖįŠŌÖŲøõĖį¼ŲČÜŅŗµÄ·“Ó¦
¢ŚŅŅĶéŌŚ¹āÕÕĢõ¼žĻĀÓėĀČĘų·“Ó¦
¢ŪŅŅĻ©Ź¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«
¢ÜŅŅĻ©Ź¹äåĖ®ĶŹÉ«
¢Ż±½ÓėÅØĻõĖįÅØĮņĖį»ģŗĻ¹²ČČÖĘČ”Ļõ»ł±½
¢ŽÓĶÖ¬ŌŚĖį»ņ¼ī“ß»ÆĢõ¼žĻĀµÄĖ®½ā·“Ó¦
¢ß±½ŌŚŅ»¶ØĢõ¼žĻĀÓėĒāĘųµÄ·“Ó¦
£ØII£©Ę»¹ūĖį 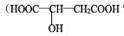 ³£ÓĆÓŚĘūĖ®”¢ĢĒ¹ūµÄĢķ¼Ó¼Į
³£ÓĆÓŚĘūĖ®”¢ĢĒ¹ūµÄĢķ¼Ó¼Į
£Ø1£©Š“³öĘ»¹ūĖįÖŠ¹ŁÄÜĶŵÄĆū³Ę_____________________
£Ø2£©Š“³öĘ»¹ūĖį·Ö±šÓėĻĀĮŠĪļÖŹ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ØÓŠ»śĪļÓĆ½į¹¹¼ņŹ½±ķŹ¾£©
¢ŁÓėNaµÄ·“Ó¦______________________________________________
¢ŚÓėNa2CO3µÄ·“Ó¦__________________________________________
£ØIII£©Š“³öŹµĻÖĻĀĮŠ×Ŗ»ÆµÄ»Æѧ·½³ĢŹ½£ØÓŠ»śĪļÓĆ½į¹¹¼ņŹ½±ķŹ¾£©
ŅŅ“¼µÄ“ß»ÆŃõ»Æ_______________________________________________
ÖĘŅŅĖįŅŅõ„___________________________________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĢģČ»Ī¬ÉśĖŲP£Ø½į¹¹ČēĻĀĶ¼£¬·Ö×Ó½į¹¹ÖŠRĪŖ±„ŗĶĢž»ł£©“ęŌŚÓŚ»±Ź÷»ØĄŁÖŠ£¬ĖüŹĒŅ»ÖÖÓŖŃųŌö²¹¼Į£¬¹ŲÓŚĪ¬ÉśĖŲPµÄŠšŹöÕżČ·µÄŹĒ 
| A£®æÉÓėäåĖ®·“Ó¦£¬ĒŅ1 moløĆĪļÖŹÓė×ćĮæäåĖ®·“Ó¦ĻūŗÄ6 mol Br2 |
| B£®æÉÓėNaOHČÜŅŗ·“Ó¦£¬1 moløĆĪļÖŹæÉÓė5 mol NaOH·“Ó¦ |
| C£®Ņ»¶ØĢõ¼žĻĀ1 moløĆĪļÖŹæÉÓėH2¼Ó³É£¬ŗÄH2×ī“óĮæĪŖ6 mol |
| D£®Ī¬ÉśĖŲPÄÜ·¢ÉśĖ®½ā·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖĪļÖŹA·Ö×ÓŹ½ĪŖC3H4O2£¬ĻŌĖįŠŌ”£FĪŖÓÉĘßøöŌ×Ó×é³ÉµÄ»·×“½į¹¹£¬·Ö×ÓŹ½ĪŖC6H8O4”£Ēėøł¾ŻŅŌĻĀæņĶ¼»Ų“šĪŹĢā£ŗ
£Ø1£©AµÄ½į¹¹¼ņŹ½ĪŖ________________________________”£
£Ø2£©·“Ó¦¢ŁµÄ·“Ó¦ĄąŠĶĪŖ__________________________”£
£Ø3£©»ÆŗĻĪļBÖŠŗ¬Ńõ¹ŁÄÜĶŵÄĆū³ĘŹĒ______________________________”£
£Ø4£©DŗĶEÉś³ÉFµÄ»Æѧ·½³ĢŹ½__________________________________”£
DŗĶE°“ÕÕ1:1·“Ó¦Ņ²æÉÉś³Éøß¾ŪĪļ£¬ĒėŠ“³öÉś³ÉøĆøß¾ŪĪļµÄ»Æѧ·“Ó¦·½³ĢŹ½=______
________________________________________________________________________ӣ
£Ø5£©GÉś³ÉHµÄ»Æѧ·½³ĢŹ½________________________________________________”£
£Ø6£©Š“³öCµÄĶ¬·ÖŅģ¹¹ĢåÖŠŹōÓŚõ„ĄąĪļÖŹµÄ½į¹¹¼ņŹ½_______________________”¢
________________________”¢____________________________________£ØÖĮÉŁŠ“3øö£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(14·Ö)¢ń”¢Ä³ŗ¬±½»·µÄ»ÆŗĻĪļA£¬ĘäĻą¶Ō·Ö×ÓÖŹĮæĪŖ104£¬Ģ¼µÄÖŹĮæ·ÖŹżĪŖ92£®3%”£
(1) AÓėäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ;
(2)ŅŃÖŖ


ĒėŠ“³öAÓėĻ””¢ĄäµÄKMnO4ČÜŅŗŌŚ¼īŠŌĢõ¼žĻĀ·“Ó¦ĖłµĆ²śĪļÓė×ćĮæŅŅĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½”” ;
(3)ŌŚŅ»¶ØĢõ¼žĻĀ£¬ÓÉA¾ŪŗĻµĆµ½µÄøß·Ö×Ó»ÆŗĻĪļµÄ½į¹¹¼ņŹ½ĪŖ”””””” ”””£
(4) AÓėĒāĘųĶźČ«¼Ó³ÉŗóµÄŅ»ĀČ“śĪļ¹²ÓŠ””””””ÖÖ”£
¢ņ”¢ÓĆČēĶ¼ĖłŹ¾×°ÖĆÖĘČ”ŅŅĖįŅŅõ„£¬Ēė»Ų“šŅŌĻĀĪŹĢā”£ 
(5)ŌŚŹŌ¹ÜÖŠÅäÖĘŅ»¶Ø±ČĄżµÄŅŅ“¼”¢ŅŅĖįŗĶÅØH2SO4µÄ»ģŗĻŅŗµÄ·½·ØŹĒ_______
(6)Ä©¶ĖøÉŌļ¹ÜµÄ×÷ÓĆŹĒ_______________”£
(7)ŹµŃé½įŹųŗó£¬Č”ĻĀŹ¢ÓŠ±„ŗĶĢ¼ĖįÄĘČÜŅŗµÄŹŌ¹Ü£¬ŌŁŃŲøĆŹŌ¹ÜÄŚ±Ś»ŗ»ŗ¼ÓČė×ĻÉ«ŹÆČļŹŌŅŗ1ŗĮÉż£¬·¢ĻÖŹÆČļ²ćĪŖČż²ć»·£¬ÓÉÉĻ¶ųĻĀŹĒŃÕÉ«ĪŖ_________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
”¾»Æѧ”Ŗ”ŖŃ”ŠŽ5£ŗÓŠ»ś»Æѧ»ł“””æ £Ø15·Ö£©
ĻĀĶ¼ÖŠ A”¢B”¢C”¢D”¢E¾łĪŖÓŠ»ś»ÆŗĻĪļ”£ŅŃÖŖ£ŗCÄÜøśNaHCO3·¢Éś·“Ó¦£¬£ĆŗĶDµÄĻą¶Ō·Ö×ÓÖŹĮæĻąµČ£¬ĒŅEĪŖĪŽÖ§Į“µÄ»ÆŗĻĪļ”£
øł¾ŻÉĻĶ¼»Ų“šĪŹĢā£ŗ
£Ø1£©ŅŃÖŖEµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ102£¬ĘäÖŠĢ¼”¢ĒāĮ½ÖÖŌŖĖŲµÄÖŹĮæ·ÖŹż·Ö±šĪŖ58.8%”¢9.8%£¬ĘäÓąĪŖŃõ£¬C·Ö×ÓÖŠµÄ¹ŁÄÜĶÅĆū³ĘŹĒ ______________£»»ÆŗĻĪļB²»ÄÜ·¢ÉśµÄ·“Ó¦ŹĒ£ØĢī×ÖÄøŠņŗÅ£©£ŗ______________
a£®¼Ó³É·“Ó¦ b£®Č”“ś·“Ó¦ c£®ĻūČ„·“Ó¦
d£®õ„»Æ·“Ó¦ e£®Ė®½ā·“Ó¦ f£®ÖĆ»»·“Ó¦
£Ø2£©·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ŹĒ__________________”£
£Ø3£©·“Ó¦¢ŚŹµŃéÖŠ¼ÓČȵÄÄæµÄŹĒ£ŗ .”£
£Ø4£©AµÄ½į¹¹¼ņŹ½ŹĒ __________________”£
£Ø5£©Š“³öĶ¬Ź±·ūŗĻĻĀĮŠČżøöĢõ¼žµÄBµÄĶ¬·ÖŅģ¹¹ĢåĖłÓŠĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½”£
¢ń£®ŗ¬ÓŠ¼ä¶žČ”“ś±½»·½į¹¹
¢ņ£®²»ŹōÓŚ·¼ĻćĖįŠĪ³ÉµÄõ„
¢ó£®Óė FeCl3ČÜŅŗ·¢ÉśĻŌÉ«·“Ó¦
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com