
������طֱ������ԡ����ԡ����������·����ķ�Ӧ���£�
MnO ��5e����8H��===Mn2����4H2O
��5e����8H��===Mn2����4H2O
MnO ��3e����2H2O===MnO2����4OH��
��3e����2H2O===MnO2����4OH��
MnO ��e��===MnO
��e��===MnO (��Һ����ɫ)
(��Һ����ɫ)
(1)�����������뷴Ӧ�п��Կ�������������ӱ���ԭ�IJ�������Һ��________Ӱ�졣
(2)��SO2ͨ����������Һ�У�������ԭ��Ӧ�����ӷ�Ӧ����Ϊ________________________________________________________��
(3)��PbO2Ͷ������MnSO4��Һ�н��裬��Һ��Ϊ�Ϻ�ɫ������˵����ȷ����________(�����)��
a�������ԣ�PbO2>KMnO4
b����ԭ�ԣ�PbO2>KMnO4
c��MnSO4��Һ�����������ữ
������(1)MnO �����������±���ԭ��Mn2���������������±���ԭ��MnO2���ڼ��������±���ԭ��MnO
�����������±���ԭ��Mn2���������������±���ԭ��MnO2���ڼ��������±���ԭ��MnO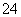 ����˸���������ӱ���ԭ�IJ�������Һ�������Ӱ�졣(2)SO2����ˮ����H2SO3�������ԣ�����������ӱ���ԭ�IJ���ӦΪMn2����(3)���������£�PbO2��Mn2���������Ϻ�ɫ��MnO
����˸���������ӱ���ԭ�IJ�������Һ�������Ӱ�졣(2)SO2����ˮ����H2SO3�������ԣ�����������ӱ���ԭ�IJ���ӦΪMn2����(3)���������£�PbO2��Mn2���������Ϻ�ɫ��MnO �������������������Դ�����������������ԣ���֪a��ȷ��b���������е�Cl�����л�ԭ�ԣ�PbO2�ܽ�Cl��������c����
�������������������Դ�����������������ԣ���֪a��ȷ��b���������е�Cl�����л�ԭ�ԣ�PbO2�ܽ�Cl��������c����
�𰸡�(1)����ԡ�(2)MnO ��5e����8H��===Mn2����4H2O��(3)a
��5e����8H��===Mn2����4H2O��(3)a


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��Ԥ�ԣ�δ���������ȼ������ɫֲ�����ֲ��Ľո�(��Ҫ�ɷ�����ά��)���ʵ��Ĵ���������ˮ��������ǣ��ٽ�������ת��Ϊ�Ҵ�������ȼ�ϡ�
(1)д������ɫֲ��ո�ת��Ϊ�Ҵ��Ļ�ѧ����ʽ��
��________________________________________________________________________��
��________________________________________________________________________��
(2)�Ҵ��������ںϳ������л����ͼ�����Ҵ�Ϊ��ʼԭ�Ϻϳɻ�״��D�Ŀ�ͼ�����ڷ�����������Ӧ�л���Ľṹ��ʽ��
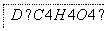
(3)д��B��C�D��D�Ļ�ѧ����ʽ��
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Կ��淴ӦaA(g)��bB(g)
 cC(g)��dD(g)���ﵽƽ��ʱ�������ʵ����ʵ���Ũ��Ӧ�������¹�ϵ��
cC(g)��dD(g)���ﵽƽ��ʱ�������ʵ����ʵ���Ũ��Ӧ�������¹�ϵ��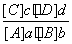 ��K��KΪһ��������Ϊ��ѧƽ�ⳣ�����䷴Ӧ��Kֵֻ���¶��йأ����з�Ӧ��CO(g)��H2O(g)
��K��KΪһ��������Ϊ��ѧƽ�ⳣ�����䷴Ӧ��Kֵֻ���¶��йأ����з�Ӧ��CO(g)��H2O(g)
 CO2(g)��H2(g)����H<0����850��ʱ��K��1��
CO2(g)��H2(g)����H<0����850��ʱ��K��1��
(1)�������¶ȵ�950�棬�ﵽƽ��K________(����ڡ�����С�ڡ����ڡ�)1��
(2)850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ����1.0 mol CO,3.0 mol H2O,1.0 mol CO2��x mol H2����
�ٵ�x��5.0ʱ������ƽ����________(�����Ӧ�����淴Ӧ��)�����ƶ���
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ�����������__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ݱ�����������M�Է��ѻ�ù���нϺõ��־����ԣ���ϳ�·������ͼ��ʾ��

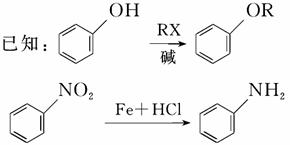
���������գ�
(1)д����Ӧ���ͣ�
��Ӧ��________����Ӧ��________��
(2)д���ṹ��ʽ��
A_____________________________________________________________��
E__________________________________________________________��
(3)д����Ӧ�ڵĻ�ѧ����ʽ��_______________________________________
_________________________________________________________________��
(4)B�ĺ������ṹ��ͬ���칹���У���һ���ܷ�������ˮ�⣬д����������ͬ���칹���еĹ�����(���ǻ�����)���Լ������ֵ�����
�Լ�(��̪����)��___________________________________________��
����_____________________________________________________��
(5)д������C�ĺ������ṹ��ֻ��4�ֲ�ͬ��ѧ������ԭ�ӵ�ͬ���칹��Ľṹ��ʽ��
_________________________________________________________
_________________________________________________________��
(6)��Ӧ�١���Ӧ�ڵ��Ⱥ�����ܵߵ��������ԭ��_________________
____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
PbO2�Ǻ�ɫ���壬���ȷֽ�ΪPb�ģ�4�ͣ�2�۵Ļ���������4�۵�Pb������Ũ��������Cl2���ֽ�1 mol PbO2���ȷֽ�õ�O2����ʣ������м���������Ũ����õ�Cl2��O2��Cl2�����ʵ���֮��Ϊ3��2����ʣ��������ɼ����ʵ���֮���� (����)��
A��1��1��ϵ�Pb3O4��PbO
B��1��2��ϵ�PbO2��Pb3O4
C��1��4��1��ϵ�PbO2��Pb3O4��PbO
D��1��4��1��ϵ�PbO2��Pb3O4��PbO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A���ܶȻ���Ļ������ܽ�ȿ϶���
B������AgCl�������Һ�м���������ˮʹAgCl�ܽ��ִﵽƽ��ʱ��AgCl���ܶȻ����䣬���ܽ��Ҳ����
C�������ܵ���ʷ��봿ˮ�У��ܽ�ﵽƽ��ʱ����������ӵ�Ũ�ȵij˻����Ǹ����ʵ��ܶȻ�
D��AgClˮ��Һ�ĵ����Ժ���������AgClΪ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ð�Һ������ӡˢп��ʱ����ϡ���ḯʴп���õ��ġ���Һ��(����������Cl����Fe3��)��ij��ѧ��ȤС�����á��ð�Һ����ȡZn(NO3)2��6H2O�Ĺ������£�

��֪��Zn(NO3)2��6H2O��һ����ɫ���壬ˮ��Һ�����ԣ�Zn(NO3)2����Ӧ���õ��IJ���������ԡ�
(1)���ð�Һ�������ʵ���Ҫ�ɷ���______(�ѧʽ����ͬ)��
(2)�ڲ������б���pH��8��Ŀ����
________________________________________________________________________��
(3)���������Ҫ�ɷ���____________��
(4)�������м�����е�Ŀ����________________���˲�����������������
________________________________________________________________________��
(5)�����ܱ���pH��2��Ŀ����____________���˲����������õ���Ҫ������
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��a��b����������ȵ�Pt�缫���AlCl3��CuSO4�Ļ����Һ[n��AlCl3����n��CuSO4��=1��9]��t1ʱ��a�缫�õ�������壬����Cl2�ڱ�״����Ϊ224 mL������������ܽ⣩��t2ʱ��Cuȫ���ڵ缫�������������ж���ȷ���� �� ��
A��a�缫���Դ�ĸ�������
B��t2ʱ�����缫���������3��84 g
C���������У���Һ��pH��������
D��t2ʱ��b�ĵ缫��Ӧ��4OH-һ4e-=2H2O+O2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У���ȷ���ǣ� ��
A��ʯ�͡�ú����Ȼ�������������ڻ�ʯȼ��
B�������£���ӦC��s����CO2��g����2CO��g�������Է����У���÷�Ӧ�Ħ�H<0
C������ͨ���ñ�ȼ���Ȼ���ֵ������ȼ��ȼ�շų������Ĵ�С��ij���ʵ���ֵԽ�������ȼ����Խ��
D�����������ͬ�������г��������NO2������Ӧ��2NO2��g��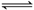
 N2O4��g�� ��H��0�������������������ɫ�Ⱥ�����������ɫ��
N2O4��g�� ��H��0�������������������ɫ�Ⱥ�����������ɫ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com