
ij��ѧѧϰ���о�С��ij�Ա����ѧϰ��֪������ˮ�е�Cl2��H2O������ѧ��Ӧ�ķ���ʽΪCl2��H2O HCl��HClO����
HCl��HClO���� ����ʾ����Cl2��H2O��Ӧ����HCl��HClO������HCl��HClO��Ӧ����Cl2��H2O��HClO����Ư���ԣ���ʹ��̪�����ָʾ������ɫ��ȥ��
����ʾ����Cl2��H2O��Ӧ����HCl��HClO������HCl��HClO��Ӧ����Cl2��H2O��HClO����Ư���ԣ���ʹ��̪�����ָʾ������ɫ��ȥ��
����Ϊ��̽�����Ʊ�����ˮ����ɺ����ʶ������˿�ѧʵ�飺�ȹ۲�����ˮ����������Ժ����ý�ͷ�ιܽ�����ˮ��ε��뺬�з�̪��NaOH��Һ�У��ߵα����������۲���������Һ�ĺ�ɫ����ȥ�����ɫ��Һ�����µ�һЩ������Ҫ��������ϻش�
(1)��ش����Ʊ�����ˮ�к�����Ԫ�ص����ʵĻ�ѧʽ��_____________________��
(2)�����ٽ��и����ʵ�飬��˵���ܿ����ж���ˮ�к���Cl2����Ҫ���ݣ�______
________________________________________________________________________��
(3)����Ԥ�⣬ʵ������Һ��ɫ��ȥ��ԭ����������֣����ü�Ҫ������˵��֮��
��________________________________________________________________________��
��________________________________________________________________________��
(4)������Ҫͨ��ʵ���һ��̽����Һ��ɫ��ȥ��ԭ���������еĢٻ��Ǣڣ��ڴ�֮ǰ������ʵ��Ԥ������ơ��������ʵ��ķ���������ͽ��ۣ�___________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ����
A�����Ȼ�����Һ�м��������ˮ��Al3����3OH��==Al(OH)3��
B������0.2 mol FeI2����Һ��ͨ��0.25 mol Cl2��8I����2Fe2����5Cl2==10Cl����4I2��2Fe3��
C��K37ClO3��Ũ����(HCl)�ڼ���ʱ����Cl2��37ClO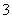 ��6HCl
��6HCl 37Cl����3Cl2����3H2O
37Cl����3Cl2����3H2O
D����0.1 mol/L�������λ�������0.1 mol/L 25 mL Na2CO3��Һ�У������Ͻ��裺2H����CO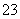 ==CO2����H2O
==CO2����H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ɸ�������ɺ����ʽ��з��ࣺ
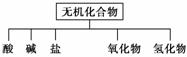
��Na��K��H��O��C��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��ڢۢĺ��档
| ���� ��� | �� | �� | �� | ������ | �⻯�� |
| ��ѧ ʽ | ��HCl ��______ | ��______ ��Ba(OH)2 | ��Na2CO3 ��______ | ��CO2 ��Na2O | ��NH3 ��H2O2 |
(1)д����ת��Ϊ�ݵĻ�ѧ����ʽ��__________________________________________
________________________________________________________________________��
(2)д��ʵ�����ɢ��Ʊ�O2�Ļ�ѧ����ʽ��___________________________________
________________________________________________________________________��
(3)ʵ�����Ʊ��߳���________��________��Ӧ�����������ķ�����____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������������ˮ������ijѧ��������ˮ�����������ʵ���Һ�������������ҩƷ���ʵ���(����)
A���Ȼ��� B��������
C��̼���� D��ʯ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������һ����Ҫ�Ļ���ԭ�ϣ����������Ʊ������Ư���⣬�������Ʊ��л��ܼ���ɱ���������ȡ������й������������У���ȷ����(����)
A����������ˮ��Һ����ͬһ�����ʣ�ֻ��״̬��ͬ
B��������������ˮ�����Կ�������ˮ���ռ�
C��������һ�ֻ���ɫ���д̼�����ζ������
D��������ˮ����AgNO3��Һʱ��ҩƷ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ʵ���������Ƴ��Ľ�����ȷ����(����)
A.Cl2��SO2����ʹƷ����Һ��ɫ,˵�����߾���������
B.��Һ�еμ��ữ��Ba(NO3)2��Һ���ְ�ɫ����,˵������Һ��һ����S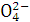
C.Fe��ϡ���ᡢϡ���ᷴӦ�������ݲ���,˵��Fe��������������û���Ӧ
D.�ֱ����HCl��NH3����ƿ������ˮ�к�Һ���Ѹ������,˵�����߾�������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ����ƺͽ����������(����)
A.����ˮ�����Һ©��,�������Ҵ�,����,�ɽ�����ȡ���Ҵ���
B.ij������ʹʪ��ĺ�ɫʯ����ֽ����,������ˮ��Һһ���Լ���
C.ij��ɫ��Һ�м�Ba(NO3)2��Һ,�ټ�ϡ����,�������ܽ�,��ԭ��Һ��һ����S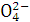
D.�ں�FeCl2���ʵ�FeCl3��Һ��ͨ������Cl2��,��ּ���,��ȥ������Cl2,���ɵõ��ϴ�����FeCl3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊȷ��ij���ȼ�(������������)����ɣ��ֱ��������ʵ�飺
(1)��ȡa g��Ʒ�������м���������NaOH��Һ��������ɵ�����(��״������ͬ)���Ϊb L����Ӧ�Ļ�ѧ����ʽ��_______________________________________________
________________________________________________________________________��
��Ʒ������������____________ g��
(2)��ȡa g��Ʒ������¼��ȣ�ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ��__________
________________________����������������������________________________��
(3)��(2)�з�Ӧ������ȴ�����������ᣬ������ɵ��������Ϊc L����������(1)����������������c��b��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���������������������Һ��Ϊ������ϡ��Ԫ�ص���ȡҺ������Ҫ�����ȡҺ�е������Ũ��Ϊ3 mol��L��1��������Ũ��Ϊ8 mol��L��1������һ��������Һ��400 L�����ⶨ���������Ũ��Ϊ12 mol��L��1�������Ũ��Ϊ1 mol��L��1��
��Ҫ�ô˻�����Һ����������ȡҺ��400 L������Һ��ϡ�Ϳ��Եõ�____L 8 mol��L��1������ᡣ��400 L������Һ�м���____L�ܶ�Ϊ1.84 g��cm��3��Ũ��Ϊ98%��Ũ���ᣬȻ��________�����ɵõ�����Ҫ�����ȡҺ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com