
���Ȼ�����(S2Cl2)�ǹ㷺������ҵ����������ӽṹ����ͼ��ʾ�������£�S2Cl2��ˮ��ˮ�⣬��������ʹƷ����ɫ�����塣����˵��������ǣ� ��
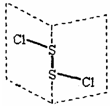
A.S2Cl2�ĽṹʽΪCl��S��S��Cl
B.S2Cl2Ϊ���м��Լ��ͷǼ��Լ��ķǼ��Է���
C.S2Br2��S2Cl2�ṹ���ƣ��۷е㣺S2Br2>S2Cl2
D.S2Cl2��H2O��Ӧ�Ļ�ѧ����ʽ����Ϊ��2S2Cl2+2H2O=SO2��+3S��+4HCl
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ȼ�����S2Cl2���ǹ㷺������ҵ��������֪���ĽṹʽΪCl�DS�DS�DCl������ˮ��Ӧ��2S2Cl2+2H2O 4HCl+SO2��+2S�����Ը÷�Ӧ����˵����ȷ���� �� ��
A��S2Cl2����������������ԭ��
B��S2Cl2ֻ��������
C��ÿ����1mol SO2ת��4mol����
D��S2Cl2ֻ����ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(08��ׯ����)���Ȼ�����S2Cl2���ǹ㷺������ҵ��������֪���ĽṹʽΪCl�DS�DS�DCl������ˮ��Ӧ��2S2Cl2+2H2O 4HCl+SO2��+2S�����Ը÷�Ӧ����˵����ȷ���ǣ� ��
A��S2Cl2����������������ԭ��
B��S2Cl2ֻ��������
C��ÿ����1mol SO2ת��4mol����
D��S2Cl2ֻ����ԭ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com