
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
£Ø1£©¢Ł¢Ś¢Ū¢Ü¢ŻĪåÖÖŌŖĖŲÖŠ£¬½šŹōŠŌ×īĒæµÄŌŖĖŲŹĒ £»£ØŠ“ŌŖĖŲĆū³Ę£©£¬øĆŌŖĖŲµÄµ„ÖŹŌŚæÕĘųÖŠČ¼ÉյĻÆѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ŌŖĖŲ¢ŁŗĶ¢ŪæÉŅŌŠĪ³ÉÖŚ¶ąµÄ»ÆŗĻĪļ£¬ĘäÖŠŗ¬ĒāĮæ×īøߵĻÆŗĻĪļŹĒ£ØŠ“»ÆѧŹ½£©
£¬ŹŌ±Č½Ļ¢ŚŗĶ¢ÜŌ×Ó°ė¾¶µÄ“󊔢Ś ¢Ü£ØĢī”°>”±»ņ”°<”±£©”£
£Ø3£©»³öŌŖĖŲ¢ÜµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼ £¬øĆŌŖĖŲµÄ×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļÄÜÓėCu·¢Éś·“Ó¦£¬·“Ó¦ÖŠ×÷Ńõ»Æ¼ĮµÄŹĒ£ØŠ“»ÆѧŹ½£© ”£
£Ø4£©Š“³öŌŖĖŲ¢ŻŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆ £¬Ę䵄֏æÉŌŚ¹¤ŅµÉĻÓėĒāŃõ»ÆÄĘČÜŅŗÖĘĘÆ°×¼Į£¬Š“³öĘä·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø5£©ŌŖĖŲ¢ŪÓė¢ŽŠĪ³É×īøß¼ŪŃõ»ÆĪļµÄµē×ÓŹ½ £¬½į¹¹Ź½ £»
£Ø6£©ŌŖĖŲ¢ßµÄŌ×ÓŠņŹżĪŖ £¬Ī»ÓŚ ×壻¢ą³ĘĪŖ Ļµ£¬ÓŠ ÖÖŌŖĖŲ”£
£Ø7£©ŌŚ±ķÖŠ»³ö½šŹōÓė·Ē½šŹō·Ö½ēĻߣ¬ŌŚ·Ö½ēĻßø½½üŅ×Ń°ÕŅ ²ÄĮĻ”£
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻņäåĖ®ÖŠ¼ÓČė×ćĮæŅŅČ©ČÜŅŗ£¬æÉŅŌ¹Ū²ģµ½äåĖ®ĶŹÉ«”£¶Ō²śÉśøĆĻÖĻóµÄŌŅņÓŠČē
ĻĀČżÖÖ²ĀĻė£ŗ¢ŁäåĖ®ÓėŅŅČ©·¢ÉśĮĖČ”“ś·“Ó¦£»¢ŚäåĖ®ÓėŅŅČ©·¢ÉśĮĖ¼Ó³É·“Ó¦¢ŪäåĖ®½«ŅŅČ©Ńõ»Æ³ÉŅŅĖį”£ĪŖĢ½¾æÄÄŅ»ÖÖ²ĀĻėÕżČ·£¬Ņ»ŃŠ¾æŠŌѧĻ°Š”×éĢį³öĮĖČēĻĀĮ½ÖÖŹµŃé·½°ø£ŗ
·½°øŅ»£ŗ¼ģŃéĶŹÉ«ŗóČÜŅŗµÄĖį¼īŠŌ
·½°ø¶ž£ŗ²ā¶Ø·“Ó¦Ē°äåĖ®ÖŠBr2µÄĪļÖŹµÄĮæŗĶ·“Ó¦ŗóBr-µÄĪļÖŹµÄĮæ
£Ø1£©·½°øŅ»ŹĒ·ńæÉŠŠ£æ £¬ĄķÓÉ
£Ø2£©¼ŁÉč·½°ø¶žÖŠ²āµĆ·“Ó¦Ē°äåĖ®ÖŠBr2µÄĪļÖŹµÄĮæĪŖa mol£¬Čō²āµĆ·“Ó¦ŗón(Br-)=
mol£¬ĖµĆ÷äåĖ®ÓėŅŅČ©·¢ÉśĮĖČ”“ś·“Ó¦£»Čō²āµĆ·“Ó¦ŗón(Br-)= mol£¬ĖµĆ÷äåĖ®ÓėŅŅČ©·¢ÉśĮĖ¼Ó³É·“Ó¦£»Čō²āµĆ·“Ó¦ŗón(Br-)= mol£¬ŌņĖµĆ÷äåĖ®½«ŅŅČ©Ńõ»Æ³ÉŅŅĖį”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĻĀĶ¼ĖłŹ¾£¬A”¢EĪŖŹÆÄ«µē¼«£¬B”¢FĪŖĢśĘ¬µē¼«”£°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā”£
 £Ø1£©“ņæŖK2£¬ŗĻ²¢K1”£BĪŖ ¼«£¬AµÄµē¼«·“Ó¦ĪŖ £¬ ×īÖÕæɹŪ²ģµ½µÄĻÖĻóŹĒ £¬
£Ø1£©“ņæŖK2£¬ŗĻ²¢K1”£BĪŖ ¼«£¬AµÄµē¼«·“Ó¦ĪŖ £¬ ×īÖÕæɹŪ²ģµ½µÄĻÖĻóŹĒ £¬
Éę¼°µÄ»Æѧ·“Ó¦·½³ĢŹ½ÓŠ£ŗ
ӣ
£Ø2£©“ņæŖK1£¬ŗĻ²¢K2”£
FĪŖ ¼«£¬F¼«µÄµē¼«·“Ó¦ĪŖ £¬
µē½ā¹ż³ĢÖŠ£¬¹Ū²ģµ½ČÜŅŗµÄĻÖĻóŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖGµÄ·Ö×Ó½į¹¹Ä£ŠĶČēÓŅĶ¼ĖłŹ¾£ØĶ¼ÖŠĒņÓėĒņÖ®¼äĮ¬Ļß±ķŹ¾µ„¼ü»ņĖ«¼ü£©£¬ÓĆ·¼Ļć
ĢžAĪŖŌĮĻŗĻ³ÉGµÄĀ·ĻßČēĻĀ£ŗ

 ?
?
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©GµÄ½į¹¹¼ņŹ½ĪŖ £¬GµÄŅ»ÖÖĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ĪŖ ”£
£Ø2£©»ÆŗĻĪļEÖŠµÄŗ¬Ńõ¹ŁÄÜĶÅÓŠ £ØĢīĆū³Ę£©”£
£Ø3£©A”ś£ĀµÄ·“Ó¦ĄąŠĶŹĒ £¬E”śFµÄ·“Ó¦ĄąŠĶŹĒ ”£
£Ø4£©ŹéŠ“»Æѧ·½³ĢŹ½
C”śD
E”śH
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
øł¾ŻµČµē×ÓŌĄķ£ŗÓɶĢÖÜĘŚŌŖĖŲ×é³ÉµÄĪ¢Į££¬Ö»ŅŖĘäŌ×ÓŹżĻąĶ¬£¬ø÷Ō×Ó×īĶā²ćµē×ÓŹż
Ö®ŗĶĻąĶ¬£¬æÉ»„³ĘĪŖµČµē×ÓĢ壬ĖüĆĒ¾ßÓŠĻąĖĘµÄ½į¹¹ĢŲÕ÷”£ŅŌĻĀø÷×éĪ¢Į£½į¹¹²»ĻąĖĘµÄ£Ø £©
A£®COŗĶN2 B£®O3ŗĶNO- C£®CO2ŗĶN2O D£®N2H4ŗĶC2H4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
1000”ꏱ£¬ĮņĖįÄĘÓėĒāĘų·¢ÉśĻĀĮŠ·“Ó¦£ŗNa2SO4(s) + 4H2(g) ![]() Na2S(s) + 4H2O(g) ”£
Na2S(s) + 4H2O(g) ӣ
£Ø1£©øĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖ____________________”£
ŅŃÖŖK1000”ę£¼K1200”ę£¬ŌņøĆ·“Ó¦ŹĒ________·“Ó¦£ØĢī”°ĪüČČ”±»ņ”°·ÅČČ”±£©”£
![]() £Ø2£©øĆĪĀ¶ČĻĀ£¬ŌŚ2LŹ¢ÓŠ1.42 g Na2SO4µÄĆܱÕČŻĘ÷ÖŠĶØČėH2ĘųĢ壬5·ÖÖÓŗó²āµĆ¹ĢĢåÖŹĮæĪŖ1.10 g”£Ōņ5·ÖÖÓÄŚH2µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ_________________”£
£Ø2£©øĆĪĀ¶ČĻĀ£¬ŌŚ2LŹ¢ÓŠ1.42 g Na2SO4µÄĆܱÕČŻĘ÷ÖŠĶØČėH2ĘųĢ壬5·ÖÖÓŗó²āµĆ¹ĢĢåÖŹĮæĪŖ1.10 g”£Ōņ5·ÖÖÓÄŚH2µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ_________________”£
£Ø3£©ÄÜĖµĆ÷øĆ·“Ó¦ŅŃ“ļµ½Ę½ŗāדĢ¬µÄŹĒ______£ØĢīŠņŗÅ£©”£
a£®ČŻĘ÷ÄŚŃ¹Ēæ±£³Ö²»±ä b£®ČŻĘ÷ÄŚĘųĢåĆܶȱ£³Ö²»±ä
c£®c(H2) = c(H2O) d£®¦ŌÕż(H2) =¦ŌÄę(H2O)
£Ø4£©ĻņĘ½ŗāĢåĻµÖŠ¼ÓČė½¹Ģ棬ĻĀĮŠĶ¼ĻńÕżČ·µÄŹĒ___________£ØĢīŠņŗÅ£©”£
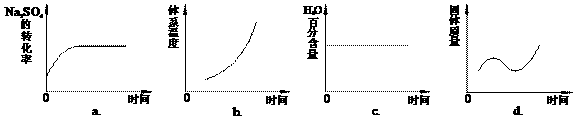
£Ø5£©ÓĆÓŠ¹ŲĄė×Ó·½³ĢŹ½ĖµĆ÷ÉĻŹö·“Ó¦²śĪļĖ®ČÜŅŗµÄĖį¼īŠŌ______________________________£¬ÓūŹ¹øĆČÜŅŗÖŠS2”ŖÅضČŌö“ó£¬Ę½ŗāÄęĻņŅĘ¶Æ£¬æɼÓČėµÄĪļÖŹŹĒ____________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹¤ŅµÖĘĀĮŹĒ²ÉÓƵē½āČŪČŚµÄŃõ»ÆĀĮµÄ·½·Ø”£ŹŌ·ÖĪöµē½āČŪČŚµÄAl2O3µÄŌĄķ£¬Š“³öÓŠ¹ŲµÄµē¼«·“Ó¦Ź½¼°×Ü·“Ó¦Ź½”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½ńÓŠNa2CO3”¢NaHCO3ŗĶNaClµÄ»ģŗĻĪļ100 g£¬¼ÓČȵ½ÖŹĮæ²»ŌŁ¼õÉŁĪŖÖ¹£¬ĘäŹ£Óą²ŠŌüĪŖ84.5 g£»½«²ŠŌüČÜÓŚĖ®£¬µĪČė×ćĮæAgNO3ČÜŅŗµĆµ½°×É«³Įµķ£¬¼Ó×ćĮæĻ”ĻõĖįŗó“ó²æ·Ö³ĮµķĻūŹ§£¬Ź£Óą³ĮµķĪŖ12.26 g”£ŹŌ¼ĘĖć»ģŗĻĪļÖŠNa2CO3”¢NaHCO3ŗĶNaClµÄÖŹĮæ·ÖŹżø÷ŹĒ¶ąÉŁ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆѧÓėÉś»īĆÜĒŠĻą¹Ų£¬ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ £Ø £©
A£®”°¼ÓĢś½“ÓĶ”±æÉÓŠŠ§Ō¤·ĄČ±ĢśŠŌʶŃŖ
B£®Ī¬ÉśĖŲC¾ßÓŠ»¹ŌŠŌ£¬ŌŚČĖĢåÄŚĘšæ¹Ńõ»Æ×÷ÓĆ
C£®µ°°×ÖŹĖ®½ā²śĪļ°±»łĖįæÉŅŌŗĻ³ÉČĖĢåĖłŠčµ°°×ÖŹ
D£®Ź³Ę·°ü×°“ü”¢Ź³Īļ±£ĻŹÄ¤µČ²ÄĮĻµÄÖ÷ŅŖ³É·ŻŹĒ¾ŪĀČŅŅĻ©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com