
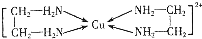 �������ڽ���Cr��Fe��Cu�ڿ�ѧ�о���ҵ�����ж�����Ҫ����;����ش��������⣺
�������ڽ���Cr��Fe��Cu�ڿ�ѧ�о���ҵ�����ж�����Ҫ����;����ش��������⣺���� ��1��������Ϊ���������������ɵ����γɵ����������ܶ���������أ������������ɵ��ӵĶ����ƶ��йأ���ɫ��Ӧ�����ԾǨ�йأ�
��2��Cr��ԭ������Ϊ24��λ�ڵ������ڣ���6��δ�ɶԵ��ӣ��������Ԫ����δ�ɶԵ�����ΪCr��һ���ҵ縺������Ԫ��NԪ�أ�
��3����ͼ��֪���������к����ۼ�����λ����SO42-��S�����¶Ե��ӣ��γ�4�����۵�����SΪsp3�ӻ�����SO42-��Ϊ�ȵ����壬��ԭ����Ϊ5���۵�����Ϊ32��
��4����ȩ�����м���̼ԭ�Ӻ���4���Ҽ���ȩ���ϵ�̼ԭ�Ӻ���3���Ҽ����ݴ��ж�̼ԭ�ӵ��ӻ���ʽ��̼ԭ���ӻ���ʽ��ͬ��������Dz�ͬ��
��5��FexO����ľ����ṹΪNaCl�ͣ�����ÿ�������к���4��Oԭ�ӣ���4����FexO�����ٸ���m=��V���㣮
��� �⣺��1��a���������ǽ��������������ɵ��Ӽ������ã���a��ȷ��
b����������ǿ�������˽�������Ӳ�ȡ��۵㣬���ܶ���������أ���b����
c�������ĵ��������������ɵ��ӵĶ����ƶ����������α��йأ���c����
d��ijЩ�����η�����ɫ��Ӧ��ԭ���Ǽ���̬���Ӵ������ϸߵĹ��ԾǨ�������ϵ��ʱ�������Կɼ������ʽ�ͷţ���d��ȷ��
�ʴ�Ϊ��ad��
��2��Cr��ԭ������Ϊ24��λ�ڵ������ڣ���̬Crԭ�ӵĺ���۵����Ų�ʽΪ3d54s1����6��δ�ɶԵ��ӣ��������Ԫ����δ�ɶԵ�����ΪCr��һ���ҵ縺������Ԫ��NԪ�أ��ʴ�Ϊ��3d54s1��N��
��3����ͼ��֪���������к����ۼ�����λ����SO42-��Sԭ�ӹµ��Ӷ���=$\frac{6+2-2��4}{2}$=0���۲���Ӷ���=4+0=0��Ϊ��������ṹ���ӻ������ĿΪ4��Sԭ���ӻ���ʽΪsp3��ԭ����Ŀ��ͬ���۵���������ͬ������Ϊ�ȵ����壬SO42-��һ�ֵȵ�����ķ�����CCl4�ȣ�
�ʴ�Ϊ�����ۼ�����λ�����������壻CCl4��
��4����ȩ�����м���̼ԭ�Ӻ���4���Ҽ���ȩ���ϵ�̼ԭ�Ӻ���3���Ҽ������Լ��е�̼ԭ�Ӳ���sp3�ӻ���ȩ���е�̼ԭ�Ӳ���sp2�ӻ���ȩ����̼ԭ�Ӳ���sp2�ӻ����Ҵ��к��д��ǻ���̼ԭ�Ӳ���sp3�ӻ���������ȩ������H-C-O�ļ��ǣ��Ҵ������е�H-C-O�ļ��ǣ�
�ʴ�Ϊ��sp3��sp2������
��5��FexO����ľ����ṹΪNaCl�ͣ�����ÿ�������к���4��Oԭ�ӣ���4����FexO��������m=��V��֪��4��$\frac{56x+16}{{N}_{A}}$=pg•cm-3����a cm��3�����x=$\frac{��{a}^{3}{N}_{A}-64}{4��56}$��
�ʴ�Ϊ��$\frac{��{a}^{3}{N}_{A}-64}{4��56}$��
���� ���⿼�龧�����㼰�ӻ�����λ���ȣ�Ϊ��Ƶ���㣬���վ���ṹ�����ӽṹ�����ʽṹ������Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬�ۺ��Խ�ǿ����Ŀ�ѶȽϴ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
| ���� | a | b | c | d | e |
| �е㣨�棩 | 58.8 | 882.9 | 444.7 | 2��355 | 1��107 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��CO2�ϳɾ�̼�����ȿɽ������ϣ��Լ��ٰ�ɫ��Ⱦ | |
| B�� | ���������������в������ﳾ���Լ��������������γ� | |
| C�� | ��ǿ������ˮ���ѵ������״������Զ���ˮ��ĸ�Ӫ���� | |
| D�� | ʹ������β�����������Լ��ٶ�����̼���ŷ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 25��ʱ����100mL��0.1mol•L-1��NH4HSO4��Һ�еμ�0.1mol•L-1��NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ����μӹ����������������������˵��������ǣ�������
25��ʱ����100mL��0.1mol•L-1��NH4HSO4��Һ�еμ�0.1mol•L-1��NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ����μӹ����������������������˵��������ǣ�������| A�� | δ�μ�NaOH��Һʱ��Һ��pHС����ͬ������0.1mol•L-1��NaHSO4��Һ��pH | |
| B�� | pHΪ7ʱ�����Һ��ˮ�ĵ���̶���� | |
| C�� | ��V��NaOH��=30mLʱ��c��NH3•H2O��+c��Na+����2c��SO42-�� | |
| D�� | �μ�NaOH��Һ�������30mL��40mL�Ĺ����У�$\frac{c��N{{H}_{4}}^{+}��}{c��{H}^{+}��}$��ֵ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
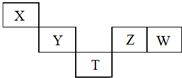 X��TԪ�������ڱ��е����λ����ͼ��ʾ������X��Y��Z��WԪ��ԭ�ӵ�����������֮��Ϊ20��TΪ��4����Ԫ�أ�����˵����ȷ���ǣ�������
X��TԪ�������ڱ��е����λ����ͼ��ʾ������X��Y��Z��WԪ��ԭ�ӵ�����������֮��Ϊ20��TΪ��4����Ԫ�أ�����˵����ȷ���ǣ�������| A�� | YW4��H2�ڸ����·�Ӧ��Ҫ����YH4��HW | |
| B�� | X��ԭ�Ӱ뾶��Tԭ�Ӱ뾶С������ԭ������֮��Ϊ29 | |
| C�� | W�������ˮ���������һ��ǿ��Z���������ˮ���� | |
| D�� | T���⻯����ܾ���ǿ�Ļ�ԭ�ԣ����ȶ���С��Z���⻯�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
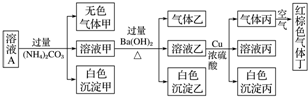
| A�� | ����Һ��һ����NO3-��Al3+��SO42-��Cl-�������� | |
| B�� | ʵ���������Cu 1.92g������������ڱ�״̬�����Ϊ448mL | |
| C�� | ������һ����BaCO3��������BaSO4 | |
| D�� | Ϊȷ��ԭ��Һ���Ƿ���Na+��K+����ͨ����ɫ��Ӧ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
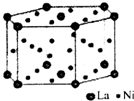 ���������ʵ���ʶԴ�ڶ���ṹ���˽⣮
���������ʵ���ʶԴ�ڶ���ṹ���˽⣮ ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��������ϩ��1mol����ȫȼ�պ����ɵ�H2OΪ2mol | |
| B�� | ���ͱ���ϩ������ʹ���Ը��������Һ��ɫ | |
| C�� | ���顢��������������������ͬ���칹�� | |
| D�� | �����2-�������һ�ȴ����Ϊ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com