
Ķ¼8-3ŹĒijĘųĢåX²śÉś²¢ŃŠ¾æĘäŠŌÖŹµÄ×°ÖĆ”£
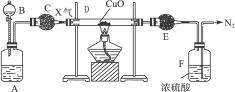
Ķ¼8-3
AÖŠŹ¢ÓŠĪ¢Čܵİ×É«¹ĢĢ壬BÖŠŹ¢ÓŠĪŽÉ«»Ó·¢ŠŌŅŗĢ壬CŗĶEÖŠŹ¢ÓŠøÉŌļ¼Į”£AŗĶBÖŠĪļÖŹĻąÓöŹ±ÓŠĪŽÉ«ĘųĢåXÉś³É£¬Ėü¾Ķ¼ÖŠŅ»ĻµĮŠ×°ÖĆŌŚÄ©¶ĖµĆµ½N2£¬ĒŅE¹ÜµÄÖŹĮæŌö¼Ó”£
£Ø1£©Š“³öŹ¢·ÅŹŌ¼ĮµÄĆū³Ę£ŗ
A.____________£¬B.____________£¬C.____________£¬E.____________”£
£Ø2£©AŗĶBÖŠĪļÖŹĻąÓöÉś³ÉXµÄÖ÷ŅŖŌŅņŹĒ£ŗ________________________”£
£Ø3£©DÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½_________________________________________”£
£Ø4£©FÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½_________________________________________”£
£Ø5£©“ÓDÖŠ·“Ó¦ĖµĆ÷ĘųĢåX¾ßÓŠ____________£ØĢī”°ĖįŠŌ”±”°¼īŠŌ”±”°Ńõ»ÆŠŌ”±»ņ”°»¹ŌŠŌ”±£©”£
£Ø1£©ÉśŹÆ»Ņ ÅØ°±Ė® ¼īŹÆ»Ņ ¼īŹÆ»Ņ
(2)CaOÓėĖ®»ÆŗĻÉś³ÉŹģŹÆ»Ņ£¬ĻūŗÄĖ®²¢·Å³ö“óĮæČČ£¬Ź¹ÅØ°±Ė®ÖŠµÄNH3ŅŻ³ö
£Ø3£©3CuO+2NH3![]() N2+3Cu+3H2O
N2+3Cu+3H2O
(4)2NH3+H2SO4====(NH4)2SO4
(5)»¹ŌŠŌ
“ÓÄ©¶ĖµÄN2ÄęĶĘæÉÖŖXÖŠŗ¬NŌŖĖŲ”£“ÓEŌöÖŲæÉĶʵ¼DÖŠ·“Ó¦ÓŠĖ®Éś³É”£N2ŗĶH2O¾łÓÉDÖŠ·“Ó¦¶ųĄ“£¬³õ²½ÅŠ¶ĻDÖŠCuOÓėŗ¬N”¢HŌŖĖŲµÄĪļÖŹX×÷ÓĆ£¬²ĀĻėXĪŖNH3”£A”¢BĻąÓöµĆNH3£¬BÓ¦Ź¢ÓŠÅØ°±Ė®£¬AÖŠÓ¦Ź¢¹ĢĢåNaOH»ņCaO£¬ŅņĢāøųĪļÖŹĪ¢ČÜ£¬æÉÖŖĖüŹĒCaO”£×īŗóČ«Ćę¼ģ²éŃéÖ¤”£


 ĢŅĄīĪÄ»ÆæģĄÖŹī¼ŁĪäŗŗ³ö°ęÉēĻµĮŠ“š°ø
ĢŅĄīĪÄ»ÆæģĄÖŹī¼ŁĪäŗŗ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijŠ£»Æѧъ¾æŠŌѧĻ°Š”×éĄūÓĆĻĀĆęĖłĢį¹©µÄŅĒĘ÷×°ÖĆŗĶŅ©Ę·ÖĘČ”NaHCO![]() ČÜŅŗ£¬Éč¼ĘČēĻĀŹµŃ锣ŹµŃéŹŅĢį¹©µÄŅ©Ę·”¢ŅĒĘ÷×°ÖĆČēĻĀ£ŗ
ČÜŅŗ£¬Éč¼ĘČēĻĀŹµŃ锣ŹµŃéŹŅĢį¹©µÄŅ©Ę·”¢ŅĒĘ÷×°ÖĆČēĻĀ£ŗ
Ņ©Ę·£ŗ¢Ł2% NaOHČÜŅŗ¢ŚĻ”HCl¢ŪĻ”H![]() SO
SO![]() ¢Ü±„ŗĶKHCO
¢Ü±„ŗĶKHCO![]() ČÜŅŗ¢ŻÅØH
ČÜŅŗ¢ŻÅØH![]() SO
SO![]() ¢ŽCaCO
¢ŽCaCO![]() ¹ĢĢå¢ßK
¹ĢĢå¢ßK![]() CO
CO![]() ·ŪÄ©
·ŪÄ©
ŅĒĘ÷×°ÖĆ£ØČēĶ¼ĖłŹ¾£©£ŗ
Ēėøł¾ŻĢāÄæŅŖĒóĶź³ÉĻĀĮŠĪŹĢā£ŗ
Ēė°“ĻĀ±ķŅŖĒó£¬ĢīŠ“Ń”ŌńµÄ×°ÖĆŗĶŅ©Ę·”£
| ·ÖĻī ÄŚČŻ | CO £ØĖęæŖĖęÓĆ£¬Ėę¹ŲĖęĶ££© £ØX£© | ³żŌÓĻ“ Ęų×°ÖĆ £ØY£© | Öʱø²ś Ę·×°ÖĆ £ØZ£© |
| Ń”ŌńµÄ×°ÖĆ £ØĢīŠņŗÅ£© | C | ||
| Ń”ŌńµÄŅ©Ę· £ØĢīŠņŗÅ£© | ¢Ł |
(2)ČēŗĪ¼ģŃéĖłŃ”ŌńµÄCO![]() ·¢Éś×°ÖĆ£ØX£©µÄĘųĆÜŠŌ£¬ĒėŠ“³öÖ÷ŅŖ²Ł×÷¹ż³Ģ£ŗ__________”£
·¢Éś×°ÖĆ£ØX£©µÄĘųĆÜŠŌ£¬ĒėŠ“³öÖ÷ŅŖ²Ł×÷¹ż³Ģ£ŗ__________”£
£Ø3£©½«×°ÖĆ°“X”¢Y”¢ZĖ³ŠņĮ¬½Ó²¢¼ģ²éĘųĆÜŠŌŗ󣬵±¼ÓČėŅ©Ę·ŹµŃ鏱£¬X×°ÖĆÖŠ·¢Éś»Æѧ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_____________£¬Y×°ÖĆÖŠ³żČ„µÄŌÓÖŹĪŖ_________________”£
£Ø4£©³£ĪĀĻĀ£¬ĻņZ×°ÖƵÄNaOHČÜŅŗÖŠĶØČė¹żĮæCO2ĘųĢ壬ĘäÄæµÄŹĒ______________”£
£Ø5£©ČōŅŖ±£Ö¤Z×°ÖĆÖŠ²»Īö³ö¾§Ģå£Ø²»æ¼ĀĒ¹ż±„ŗĶČÜŅŗĪŹĢā£©£¬NaOHČÜŅŗ×ī“óÅØ¶Č²»Äܳ¬¹ż________________%£ØÖŹĮæ·ÖŹż£©”£
ø½£ŗÓŠ¹ŲĪļÖŹŌŚ³£ĪĀ£Ø25 ”ę£©Ź±µÄČܽā¶Č
| »ÆѧŹ½ | Na | NaHCO | NaOH | NaCl | Na |
| Čܽā¶Č £Øg/100 g H | 21.3 | 9.60 | 107 | 35.8 | 19.4 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com