
| ���� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| �� | �� | �� | ||||||
| �� | �� | �� | �� | �� | �� | �� | ||
| �� | �� | �� |

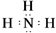 ��������������Ķ�Ӧ��ˮ����ĵ���ʽ
��������������Ķ�Ӧ��ˮ����ĵ���ʽ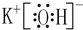 ��
�� ��
�� ���� ��Ԫ�������ڱ��е�λ�ÿ�֪����ΪN����ΪF����ΪNa����ΪMg����ΪAl����ΪSi����ΪCl����ΪAr����ΪK����ΪBr��
��1��ͬһ�����У�ԭ������Խ��ԭ�Ӱ뾶ԽС���ݴ��ж�ԭ�Ӱ뾶�����Ԫ�أ���д�������ӽṹʾ��ͼ��
��2��Ԫ�ؽ�����Խǿ������������Ӧˮ����ļ���Խǿ��Ԫ�طǽ�����Խǿ��������������Ӧˮ���������Խǿ��ע��FԪ��û����������ϼۣ��ݴ˽����жϣ�
��3���ٵ��⻯�ﰱ��������Ϊ���ۻ���������к���3��N-H����N�����ﵽ8�����ȶ��ṹ��������������Ķ�Ӧ��ˮ����Ϊ�������أ���������Ϊ���ӻ���������ʽ����Ҫ�����������������ɣ����������ӻ���Ҫ����������ӣ�
��4����ΪCl����ΪBr������λ��ͬһ���壬ԭ������Խ�ǽ�����Խ������Ӧ���ʵĻ�����Խ��������ͨ���û���Ӧ�Ƚ϶��ߵĻ����ԣ�
��5���ܺ͢��γɵĻ������Ȼ�þ���Ȼ�þ�������ӻ�����õ���ʽ��ʾ�������γɹ���ʱ����ͷ���Ϊԭ�ӵĵ���ʽ����ͷ�ұ�Ϊ���������ʽ��
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪN����ΪF����ΪNa����ΪMg����ΪAl����ΪSi����ΪCl����ΪAr����ΪK����ΪBr��
��1���ۡ���Ԫ��λ��ͬһ���ڣ�ͬһ������ԭ������Խ��ԭ�Ӱ뾶ԽС����ԭ�Ӱ뾶�����ΪNa�������ӵĺ˵����Ϊ11�������������Ϊ10�����������8�����ȶ��ṹ�������ӽṹʾ��ͼΪ�� ��
��
�ʴ�Ϊ��Na�� ��
��
��2���١���Ԫ���зǽ�������ǿ��ΪF������Fû����ۺ����ᣬ���ڵڶ�λ��ΪCl����Ԫ�ص�����������Ӧ��ˮ������������ǿ����HClO4��10��Ԫ���У���������ǿ��ΪK���������ǿ����KOH��
�ʴ�Ϊ��HClO4��KOH��
��3����ΪNԪ�أ����⻯��Ϊ����������Ϊ���ۻ���������к���3��N-H���������ʽΪ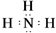 ��
��
��ΪKԪ�أ������������Ķ�Ӧ��ˮ����ΪKOH����������Ϊ���ӻ���������ʽΪ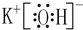 ��
��
�ʴ�Ϊ��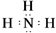 ��
�� ��
��
��4��������γɵĵ��ʷֱ�ΪCl2��Br2���������Խ�ǿ��ΪCl2��ͨ���û���ӦCl2+2NaBr=2NaCl+Br2��˵������ʵ��
�ʴ�Ϊ��Cl2��Cl2+2NaBr=2NaCl+Br2��
��5���ܺ͢��γɵĻ�����ΪMaCl2���Ȼ�þΪ���ӻ�����õ���ʽ��ʾ�Ȼ�þ���γɹ���Ϊ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼����λ�á��ṹ�����ʵĹ�ϵ����Ŀ�Ѷ��еȣ���ȷԭ�ӽṹ��Ԫ�������ɡ�Ԫ�����ڱ��Ĺ�ϵΪ���ؼ���ע�����ճ�����ѧ����ĸ����дԭ������������ѧ���ķ������������Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ӳɷ�Ӧ | B�� | �ۺϷ�Ӧ | C�� | ������Ӧ | D�� | ˮ�ⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������=A-n | B�� | ������=A-Z | C�� | ������=Z+n | D�� | ���������=n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ɫ��Һ�п��ܴ�������Al3+��NH4+��Cl?��H+ | |
| B�� | ������Һ�п��ܴ�������Na+��ClO?��SO42?��I? | |
| C�� | ������Һ�п��ܴ�������Na+��K+��Cl?��HCO3? | |
| D�� | ������Һ�п��ܴ�������Fe3+��K+��Cl?��I? |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| װ�� | ��ʢҩƷ | ʵ������ | ���ۻ���� |
| B | �� | �� | �� |
| C | CuO���� | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
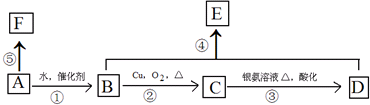
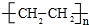 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
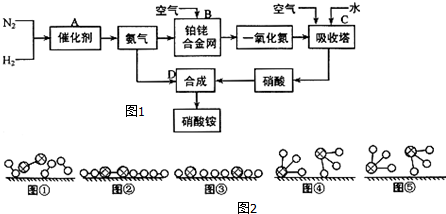
 ��
�� ��
��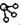 �ֱ��ʾH2��N2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں͢ۺ���ֱ���N2��H2�������ڴ������桢�ڴ������棬N2��H2�л�ѧ�����ѣ�
�ֱ��ʾH2��N2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں͢ۺ���ֱ���N2��H2�������ڴ������桢�ڴ������棬N2��H2�л�ѧ�����ѣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2��10-3 | B�� | 2��10-2 | C�� | 2��10-1 | D�� | 2��10-4 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com