
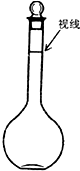
 ʵ��������500mL��0.2mol/L��Na2SO4��Һ��ʵ����������У�
ʵ��������500mL��0.2mol/L��Na2SO4��Һ��ʵ����������У����� ��1���ù�������һ�����ʵ���Ũ����Һ��һ�㲽��Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ����ǩ���ݴ�����
��2���������ƶ������ʵ���Ũ����Һһ�㲽��ѡ����Ҫ��������
��� �⣺��1���ù�������һ�����ʵ���Ũ����Һ��һ�㲽��Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ����ǩ��������ȷ��˳��Ϊ���٢ڢܢۢݣ�
�ʴ�Ϊ���٢ڢܢۢݣ�
��2���ù�������һ�����ʵ���Ũ����Һ��һ�㲽��Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ����ǩ���õ���������������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�
���Ի�ȱ�ٵ���������������500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ����������500mL����ƿ����ͷ�ιܣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ�������������ǽ���ؼ���ע������ƿ����ѡ����Ŀ�ѶȲ���


 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��Ӧʱ��/min | n��SO2��/mol | n��O2��/mol |
| 0 | 0.10 | 0.060 |
| t1 | 0.012 | |
| t2 | 0.016 |
| A�� | ��Ӧ��0��t1 min�ڵ�ƽ������Ϊv��SO3��=0.088/t1 mol•L-1•min-1 | |
| B�� | ���������������䣬��ʼʱ�������г���0.10molSO3��0.010 molO2������ƽ��ʱ��n��SO2��=0.012 mol | |
| C�� | ���������������䣬�����¶ȣ�ƽ��ʱc��SO2��=0.0070mol•L-1����Ӧ�ġ�H��0 | |
| D�� | ��ͬ�¶��£���ʼʱ�������г���0.050molSO2��0.030molO2���ﵽƽ��ʱSO2ת���ʴ���88% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 �����������[HPO��OC2H5��2]Ϊ��Ҫ����ȼ���ܼ���ʵ���Ҳ������Ȼ�����ˮ�Ҵ��Ʊ��������������PCl3+4C2H5OH��HPO��OC2H5��2+HCl��+2C2H5Cl+H2O��ʵ�鲽�����£�
�����������[HPO��OC2H5��2]Ϊ��Ҫ����ȼ���ܼ���ʵ���Ҳ������Ȼ�����ˮ�Ҵ��Ʊ��������������PCl3+4C2H5OH��HPO��OC2H5��2+HCl��+2C2H5Cl+H2O��ʵ�鲽�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ڿ�����ȼ�� | B�� | ��Br2����ȡ����Ӧ | ||
| C�� | ��H2�����ӳɷ�Ӧ | D�� | ��ʹ���������Һ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com