

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
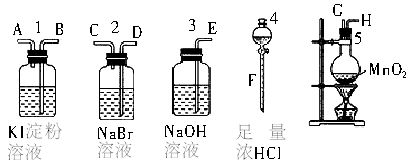
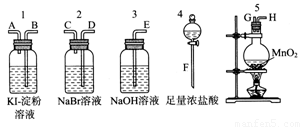
��10�֣�Ϊ�˱Ƚ�±�ص��ʵ�������ǿ��������ʵ��������ȡCl2(����MnO2��Ũ���ᷴӦ����ȡCl2)������Cl2����ͨ��NaBr��Һ��KI��������Һ�С���ͼ��ʾ������ҩƷ���Իش�
(1)������ȡ���������������ʱ�����������ӿڵ�����˳��Ϊ________��________��________��________��________��________��________��________��������ĸ��
(2)ʵ�鿪ʼ��װ��5�з�Ӧ�����ӷ���ʽΪ______________________________��
(3)װ��3��������__________________________________����Ӧ�����ӷ���ʽΪ_______________________________��
(4)װ��1�в����������ǣ���Һ�ȱ���һ��ʱ�����ɫ��ȥ����������ǿ�ᣬ��д����ɫ��ȥ�Ļ�ѧ����ʽ��___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�����ص�һ��ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
Ϊ�˱Ƚ�±�ص��ʵ�������ǿ������ʵ��������ȡCl2(����MnO2��Ũ���ᷴӦ����ȡCl2)����Cl2����ͨ��NaBr��Һ��KI������Һ�С���ͼ��ʾ������ҩƷ�Իش�?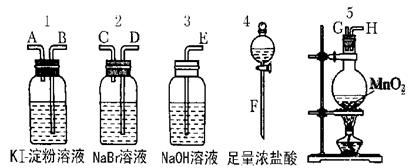
(1)������ȡ���������������ʱ���������ӿڵ�����˳��ΪF��G��H��______��______��______��A��E��
(2)ʵ�鿪ʼ��װ��5�з�Ӧ�Ļ�ѧ����ʽΪ______________________��
(3)װ��3��������________________________
��Ӧ�����ӷ���ʽΪ______________________________________��
(4)װ��1�в�����������___________ ��Ӧ�����ӷ���ʽΪ____________________��
��5���������ӵĻ�ԭ�����ڵ����ӵĻ�ԭ�ԣ�ͨ��ʵ�飬±�ص��ʵ���������ǿ������˳��Ϊ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�����н�����и�һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��10�֣�Ϊ�˱Ƚ�±�ص��ʵ�������ǿ��������ʵ��������ȡCl2(����MnO2��Ũ���ᷴӦ����ȡCl2)������Cl2����ͨ��NaBr��Һ��KI��������Һ�С���ͼ��ʾ������ҩƷ���Իش�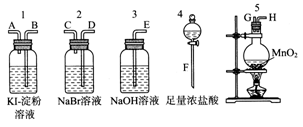
(1)������ȡ���������������ʱ�����������ӿڵ�����˳��Ϊ________��________��________��________��________��________��________��________��������ĸ��
(2)ʵ�鿪ʼ��װ��5�з�Ӧ�����ӷ���ʽΪ______________________________��
(3)װ��3��������__________________________________����Ӧ�����ӷ���ʽΪ_______________________________��
(4)װ��1�в����������ǣ���Һ�ȱ���һ��ʱ�����ɫ��ȥ����������ǿ�ᣬ��д����ɫ��ȥ�Ļ�ѧ����ʽ��___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��㶫ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
Ϊ�˱Ƚ�±�ص��ʵ�������ǿ������ʵ��������ȡCl2(����MnO2��Ũ���ᷴӦ����ȡCl2)����Cl2����ͨ��NaBr��Һ��KI������Һ�С���ͼ��ʾ������ҩƷ�Իش�?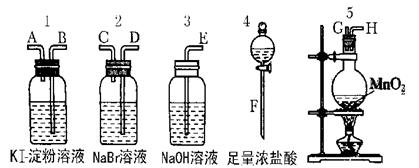
(1)������ȡ���������������ʱ���������ӿڵ�����˳��ΪF��G��H��______��______��______��A��E��
(2)ʵ�鿪ʼ��װ��5�з�Ӧ�Ļ�ѧ����ʽΪ______________________��
(3)װ��3��������________________________
��Ӧ�����ӷ���ʽΪ______________________________________��
(4)װ��1�в�����������___________ ��Ӧ�����ӷ���ʽΪ____________________��
��5���������ӵĻ�ԭ�����ڵ����ӵĻ�ԭ�ԣ�ͨ��ʵ�飬±�ص��ʵ���������ǿ������˳��Ϊ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ��ʡ�����и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��10�֣�Ϊ�˱Ƚ�±�ص��ʵ�������ǿ��������ʵ��������ȡCl2(����MnO2��Ũ���ᷴӦ����ȡCl2)������Cl2����ͨ��NaBr��Һ��KI��������Һ�С���ͼ��ʾ������ҩƷ���Իش�
(1)������ȡ���������������ʱ�����������ӿڵ�����˳��Ϊ________��________��________��________��________��________��________��________��������ĸ��
(2)ʵ�鿪ʼ��װ��5�з�Ӧ�����ӷ���ʽΪ______________________________��
(3)װ��3��������__________________________________����Ӧ�����ӷ���ʽΪ_______________________________��
(4)װ��1�в����������ǣ���Һ�ȱ���һ��ʱ�����ɫ��ȥ����������ǿ�ᣬ��д����ɫ��ȥ�Ļ�ѧ����ʽ��___________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com