
����Ŀ��������������־������־�����������й��ڰ�ͭ�ļ��أ���������ͭ��ͭ���Ͻ��������⣬����Ҫ������ң������������������Ʒ���ش��������⣺
��1����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ______��3d�ܼ��ϵ�δ�ɶԵ�����Ϊ_______��
��2�����������ڰ�ˮ�γ�[Ni(NH3)6]SO4��ɫ��Һ��
��[Ni(NH3)6]SO4�������ӵ����幹����________��
����[Ni(NH3)6]SO4��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ______���ṩ�µ��ӶԵijɼ�ԭ����______��
�۰��ķе�_______������ڡ����ڡ���좣�PH3����ԭ����_______������______���ӣ�����ԡ��Ǽ��ԡ���������ԭ�ӵĹ���ӻ�����Ϊ_______��
��3��ͭ����̼ͭԭ�ӵĶѻ���ʽ��ͼ��ʾ��
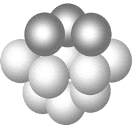
�ٻ�̬ͭ��Ԫ�����ڱ���λ��__________________��
��ÿ��ͭԭ����Χ���������ͭԭ����Ŀ_________��
(4)ijMԭ�ӵ���Χ�����Ų�ʽΪ3s23p5��ͭ��M�γɻ�����ľ�����ͼ��ʾ(�ڵ����ͭԭ��)��
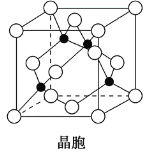
�ٸþ���Ļ�ѧʽΪ__________________��
����֪ͭ��M�ĵ縺�Էֱ�Ϊ1.9��3.0����ͭ��M�γɵĻ���������________(����ӡ����ۡ�)�����
����֪�þ�����ܶ�Ϊ�� g��cm��3�������ӵ�����ΪNA����þ�����ͭԭ�Ӻ�Mԭ��֮�����̾���Ϊ________pm(ֻд����ʽ)��
���𰸡�1s22s22p63s23p63d84s2 2 �������� ��λ�� Nԭ�� ���� �����Ӽ������� ���� sp3�ӻ� ��������IB�� 12 CuCl ���� ![]() ��
��![]() 1010
1010
��������
��1����Ԫ�ػ�̬ԭ������Ϊ28�������Ų�ʽ���������ȷ��δ�ɶԵ�����Ŀ��
��2����������Ϊ��������ӣ������ӻ���ʽȷ���ռ乹�ͣ�
��Ni2+��NH3֮���γɵĻ�ѧ��Ϊ��λ����Nԭ���ṩ�µ��ӶԳɼ���
�۰����Ӽ����������������ȷ��Ӽ��������ǿ��
��3����ͭ��29��Ԫ��ȷ���������ڱ��е�λ�ã�
��ͭ�ľ������������������ܶѻ���
(4)Mԭ�ӵ���Χ�����Ų�ʽΪ3s23p5����Mԭ��ΪCl��
�ٸ��ݾ���ͼ��̯����⣻
�������ɼ�Ԫ��ԭ�Ӽ�ĵ縺�Բ�ֵ����1.7�γ����Ӽ���С��1.7�γɹ��ۼ������жϣ�
�۸þ�����������ʯ�ľ�����Cu��Cl����ľ�������Խ��ߵ�1/4�������ܶ�����ⳤ���������뼴�ɣ�
��1����Ԫ�ػ�̬ԭ������Ϊ28����������Ų�ʽΪ1s22s22p63s23p63d84s2��3d�ܼ���5��������Ų�8�����ӣ�����2��δ�ɶԵ��ӣ�
��2����������Ϊ��������ӣ�������ԭ�ӹµ��Ӷ���=![]() ��6+2-2��4��=0����4�����ۼ���Ϊsp3�ӻ����ռ乹��Ϊ�������幹�ͣ�
��6+2-2��4��=0����4�����ۼ���Ϊsp3�ӻ����ռ乹��Ϊ�������幹�ͣ�
��Ni2+��NH3֮���γɵĻ�ѧ��Ϊ��λ����Nԭ���ṩ�µ��ӶԳɼ���
�۰����Ӽ�����������좷��Ӽ䲻����������ʰ����ӵķе����좣�PH3����������Ϊ�ɼ��Լ��γɵļ��Է��ӣ����ĵ�ԭ��Ϊsp3�ӻ���
��3����ͭ��29��Ԫ�أ�λ�ڵ�������IB�壻
��ͭ�ľ������������������ܶѻ����ѻ���ʽABC�������λ��Ϊ12��ÿ��ͭԭ����Χ�����ͭԭ����Ŀ��12��
(4)Mԭ�ӵ���Χ�����Ų�ʽΪ3s23p5����Mԭ��ΪCl��
�ٸ��ݾ���ͼ���֪��ͭԭ�������ڣ���4����Clԭ���ڶ�������ģ���8��1/8+6��1/2=4������ԭ�ӵĸ�����Ϊ1:1����ѧʽΪCuCl��
��һ����Ϊ�����ɼ�Ԫ��ԭ�Ӽ�ĵ縺�Բ�ֵ����1.7�γ����Ӽ���С��1.7�γɹ��ۼ���ͭ��Cl�縺�Բ�ֵ=3.0-1.9=1.1��1.7�����ڹ��ۻ����
�۾��������V=��64+35.5����4g/����g/cm��NA���������ⳤ=![]() cm���þ�����������ʯ�ľ�����Cu��Cl����ľ�������Խ��ߵ�1/4������Ϊ
cm���þ�����������ʯ�ľ�����Cu��Cl����ľ�������Խ��ߵ�1/4������Ϊ![]() ��
��![]() 1010pm��
1010pm��


 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Fe2(SO4)3��Һ�����ڸ�ʴ��Ե���ϵ�ͭ��������ӡˢ��·�塣�������һ���������£��Ӹ�ʴ��ķ�Һ(��Ҫ��Fe3+��Fe2+��Cu2+)�л���ͭ�������»��Fe2(SO4)3��Һ��
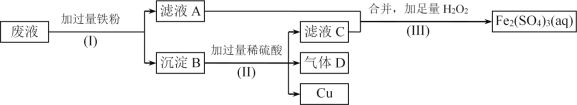
(1)����(I)�з����������Ϊ____________________��
(2)����B����Ҫ�ɷ���____________________������D�ĵ���ʽΪ__________��
(3)д������(III)�з�����Ӧ�����ӷ���ʽ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ����������(��Ҫ�ɷ�ΪAl2O3����Fe2O3����)Ϊԭ��ұ�����Ĺ����������£�
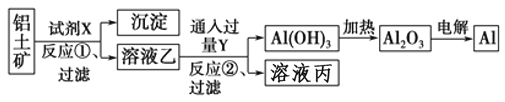
����������ȷ����
A. ���������̣��Լ�X����������������Һ��Ҳ����������
B. ��Ӧ�ٹ��˺����ó���Ϊ������
C. ͼ�����е�ת����Ӧ������������ԭ��Ӧ
D. ��Ӧ�ڵ����ӷ���ʽΪ2AlO2����CO2��3H2O��2Al(OH)3����CO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������У�ǰ�߸պ��Ǻ����������� (����)
A. 2 mol H2O��Ħ��������1 mol H2O��Ħ������
B. 200 mL 1 mol��L-1�Ȼ�����Һ��c(Cl-)��100 mL 2 mol��L-1�Ȼ�����Һ��c(Cl-)
C. 64 g������������ԭ�����ͱ�״����22.4 Lһ����̼����ԭ����
D. 20% NaOH��Һ��NaOH�����ʵ���Ũ�Ⱥ�10% NaOH��Һ��NaOH�����ʵ���Ũ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
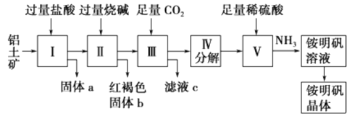
����Ŀ��������Ҫ�Ľ������ϣ�������(��Ҫ�ɷ���Al2O3��������SiO2��Fe2O3����)�ǹ�ҵ����ȡ����ԭ�ϡ�ʵ����ģ�ҵ����������Ϊԭ�������������[NH4Al(SO4)2]�Ĺ���������ͼ��ʾ��
��ش��������⣺
(1)����a�Ļ�ѧʽΪ________������b��ѧʽΪ________������ͨ������CO2���巢����Ӧ�����ӷ���ʽΪ________________��
(2)������ȡ�������Һ�Ļ�ѧ����ʽΪ__________________��
(3)���������������Ҫ�õ��ķ��뷽����__________________�����������Һ�������������IJ�����__________________��
(4)��������������������������ƣ��ټ���������ᣬ��ʱ���ȳ��ֵĹ���a��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����100mL MgCl2��AlCl3�Ļ����Һ�У���μ���NaOH��Һֱ�����������ⶨ������NaOH���ʵ��������ó��������ʵ����Ĺ�ϵ��ͼ��ʾ���ش��������⡣
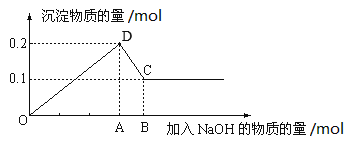
(1)ͼ��CD�η�Ӧ�Ļ�ѧ����ʽΪ___________��C����ڵĹ�������Ϊ________��
(2)A�������Ϊ_______�����Һ�У�MgCl2��Ũ��Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������H����Ҫ���л������E��F��һ�������ºϳɣ������ַ�Ӧ������ʡ�ԣ�����ע���ͷ��ָ��
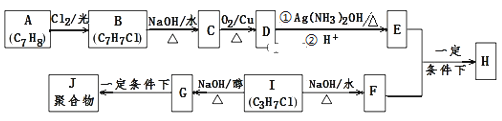
��֪������Ϣ��
i��A���ڷ�������H����������
ii��I�ĺ˴Ź�������Ϊ����壬�ҷ�������Ϊ6��1��
�ش��������⣺
��1��E�ĺ��������������� ______ ��B�Ľṹ��ʽ��___________________��
��2��B��C��G��J�����ķ�Ӧ���ͷֱ�Ϊ ___________��____________��
��3����E+F��H�Ļ�ѧ����ʽ��____________________________________��
��D��������Һ��Ӧ�Ļ�ѧ����ʽ��__________________________________��
��4��E��ͬϵ��K��E��Է���������28����K��ͬ���칹�干 ______ �֣����к˴Ź���������ʾΪ4��壬�ҷ����֮��Ϊ6:2:1:1��д������Ҫ��ĸ�ͬ���칹��Ľṹ��ʽΪ��д��1�ּ��ɣ� ____________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����120 ��ʱ����a L��ϩ��b L���顢c L��Ȳ(b>c)��d L����(����)���(a+b+c+d=25)����ȼʹ֮���ȼ�պָ���ԭ�����¶ȣ�������������������
A. 10 L B. 15 L C. 25 L D. 27 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��Ϊ̽��Cl2��Br2��Fe3+��������ǿ�������������ʵ�飺
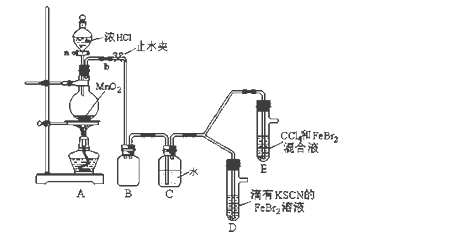
(1)��װ��A�з�����Ӧ�����ӷ���ʽ��_____________________��
������ʵ��װ�ô���һ�����ԵIJ��㣬��ָ��_______________________��
(2)�ø������װ�ý���ʵ�飬ʵ��������£�
ʵ����� | ʵ������ | ���� |
����a����Բ����ƿ�е�������Ũ���Ȼ��رջ���a����ȼ�ƾ��ơ� | Dװ���У���Һ��� Eװ���У�ˮ����Һ��ƣ� ���²�CCl4�������Ա仯�� | Cl2��Br2��Fe3+����������ǿ������˳��Ϊ�� ________________________ |
(3)��æ�ڹ۲�ͼ�¼��û�м�ʱֹͣ��Ӧ��D��E�о��������µı仯��
Dװ���У���ɫ������ȥ��
Eװ���У�CCl4��������ɫ��Ϊ��ɫ������ɫ���ֱ����ɺ�ɫ��
Ϊ̽������ʵ������ı��ʣ�С��ͬѧ����������£�
����Fe3+ +3SCN��![]() Fe(SCN)3 ��һ�����淴Ӧ��
Fe(SCN)3 ��һ�����淴Ӧ��
����(SCN)2������±�ص������ơ������ԣ�Cl2 �� (SCN)2 ��
����Cl2��Br2��Ӧ����BrCl��BrCl�ʺ�ɫ(�Դ���ɫ)���е�Լ5�棬����ˮ�ܷ���ˮ�ⷴӦ���Ҹ÷�ӦΪ��������ԭ��Ӧ��
����AgClO��AgBrO��������ˮ��
������ƽ���ƶ�ԭ��(�����������)����Cl2����ʱD����Һ��ɫ��ȥ��ԭ��___________________________________________������Ƽ�ʵ��֤���������ͣ�ȡ������ɫ�����Һ���μ�______________��Һ������Һ��ɫ________�������������Ǻ����ġ�
����̽��E����ɫ�仯��ԭ�����ʵ�����£�
�÷�Һ©�������E���²���Һ�������ռ���ɫ���ʣ�ȡ����������AgNO3��Һ������۲쵽���а�ɫ��������������������������������ʽ(���ӻ�ѧ����ʽ����)���Ͳ�����ɫ����ԭ��__________________��_______________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com