
| m |
| M |
| V |
| Vm |
| n |
| V |
| 73g |
| 36.5g/mol |
| 73g |
| 1000g+73g |
| 500L |
| 22.4L/mol |
| 500 |
| 22.4 |
| ||
| 1L |


 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д� ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
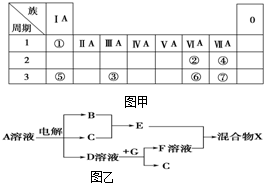 ��ͼ��ΪԪ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã����û�ѧ����ش��������⣺
��ͼ��ΪԪ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã����û�ѧ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
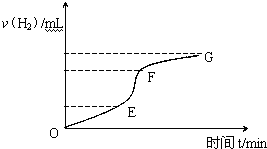 �ô�����п����ϡ���ᷴӦ��ȡ�������壬��ش�
�ô�����п����ϡ���ᷴӦ��ȡ�������壬��ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| SO | 2- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��һ���¶��£��������ˮϡ�����У���Һ�ĵ���������ͼ��
��һ���¶��£��������ˮϡ�����У���Һ�ĵ���������ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ���� | �����Ũ��/mol?L-1 | �ζ����ʱ���� ���������/mL | ����NaOH��Һ �����/mL |
| 1 | 0.1152 | 26.72 | 25.00 |
| 2 | 0.1152 | 29.02 | 25.00 |
| 3 | 0.1152 | 26.70 | 25.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����1����3�� |
| B����1����4�� |
| C����1����3����4�� |
| D����2����3����4�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com