


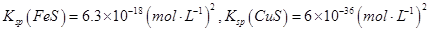
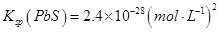
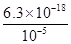 ��6.3��10��13mol/L��
��6.3��10��13mol/L��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ��ѧʽ | ���볣��(25��) |
| CH3COOH | Ki =1.7��10��5 |
| HClO | Ki =3.0��10��8 |
| H2CO3 | Ki1=4.3��10��7 Ki2=5.6��10��11 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.1mo1/L NaI��Һ��K+��Na+��MnO4-��OH- |
| B�����ܽ�CaCO3����Һ��K+��NH4+��Cl-��NO3- |
| C��0.1mo1/LNaHSO3��Һ��Na +��Mg2+��SO42-��ClO- |
| D��c(H+)/c(OH-)=1013������Һ��K+��Fe2+��Cl-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������KSCN�Ժ�ɫ����Һ�У�K+��NH4+��Cl�D��I�D |
| B������84������ҹ����Ч�ɷ�NaClO������Һ�У�Fe2+��Cl�D��Ca2+��Na+ |
| C�������£�pH=1����Һ�У�NH4+��Na+��Cl�D��Cu2+ |
| D�������£�pH=13����ɫ��Һ�У�K+��NO3�D��HCO3�D��Al3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c (Na��)="c" (HCO3��) + c (CO32��) + c (H2CO3) |
| B���¶����ߣ���Һ��pH���ߣ�����Һ�е�c��H������c��OH�����˻����� |
| C����������Ũ�ȵ�CH3COOH��Һ��Ӧ����Һ��c��Na������c��CH3COO���� |
| D������������NaOH���壬��Һ��pH��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��K+��Na+��NO3-��SiO32- | B��Al3+��K+��SO42-��S2- |
| C��Ag+��Na+��NH3��H2O��NO3- | D��Cu2+��Fe2+��Cl-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | �� �� | Ҫ �� |
| A | NH��Al3����SO��H�� | �μ�NaOH��Һ������������� |
| B | K����NO��Cl����HS�� | c(K��)��c(Cl��) |
| C | Fe2����NO��SO��Cl�� | ��εμ�����ʱ��Һû����ɫ�仯 |
| D | Na����HCO��Mg2����SO | �μӰ�ˮ�����г������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c(H+)> c(F-) | B��c(H+)> c(HF) |
| C��c(OH-)> c(HF) | D��c(HF) > c(F-) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NH4+��K+��OH-��SO42- | B��Na+��Fe2+��NO3-��Cl- |
| C��Ca2+��Cl-��HCO3-��K+ | D��K+��Na+��Br-��SO42- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com