
��Ҷ�к��ж������������彡�����л��ɷּ��ơ�����������Ԫ�أ�ij��ѧ�о���ѧϰС����Ʒ������ԲⶨijƷ�Ʋ�Ҷ�и�Ԫ�ص�����������������Ԫ�صĴ���(��֪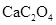 Ϊ��ɫ��������)������ȡ200g��Ҷ��Ʒ���յûҷۺ�������²�����
Ϊ��ɫ��������)������ȡ200g��Ҷ��Ʒ���յûҷۺ�������²�����
��ش������й����⣺
(1)����������ʾ��ijЩ�������ӵ�����������ȫ������pHΪ����
| ���� |
|
|
| ��ȫ����ʱ��pH | 13 | 4.1 |
ʵ��ǰҪ�Ƚ���Ҷ��Ʒ�������ճɻҷۣ�����ҪĿ����__________��
(2)д������ҺA������D�����ӷ�Ӧ����ʽ__________��
(3)Ϊ��֤ʵ�龫ȷ�ȣ�����D��E��Ҫ�ֱ�ϴ�ӣ�����ϴ��Һת�ƻ�ĸҺ�У���
�жϳ���D�Ѿ�ϴ�Ӹɾ��ķ�����__________��
(4)��KMnO![]() ����Һ�ζ�C��Һʱ�������ķ�ӦΪ��
����Һ�ζ�C��Һʱ�������ķ�ӦΪ��
![]() ��
��
�ֽ���ҺCϡ����500 mL����ȡ���е�25.00 mL��Һ���������ữ����0��1000
mol��L-1��KMnO4����Һ�ζ����յ�ʱ����KMnO4��Һ10.00 mL��
�ٴ˲�������������Ҫ�õ�������Щ����(��д���)________��
�ڴﵽ�ζ��յ�ʱ����Һ����ɫ�仯��________��
�۵ζ����յ㣬���ú�����ͼ��ȡKMnO4����Һ�Ŀ̶����ݣ���ⶨ�ĸ�Ԫ�غ�����________(�ƫ�ߡ�����ƫ�͡�������Ӱ�족)��
(5)ԭ��Ҷ�и�Ԫ�ص���������Ϊ________��
(6)����ͨ��������ҺA����֤��Ʒ�Ʋ�Ҷ���Ƿ�����Ԫ�أ������Լ���ʵ��������________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҷ�к��ж������������彡�����л��ɷּ��ơ�����������Ԫ�أ�ij��ѧ�о���ѧϰС����Ʒ������ԲⶨijƷ�Ʋ�Ҷ�и�Ԫ�ص�����������������Ԫ�صĴ���(��֪![]() Ϊ��ɫ��������)������ȡ200g��Ҷ��Ʒ���յûҷۺ�������²�����
Ϊ��ɫ��������)������ȡ200g��Ҷ��Ʒ���յûҷۺ�������²�����
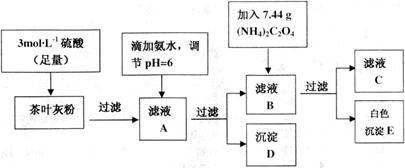
��ش������й����⣺
(1)����������ʾ��ijЩ�������ӵ�����������ȫ������pHΪ����
| ���� |
|
|
| ��ȫ����ʱ��pH | 13 | 4.1 |
ʵ��ǰҪ�Ƚ���Ҷ��Ʒ�������ճɻҷۣ�����ҪĿ����__________��
(2)д������ҺA������D�����ӷ�Ӧ����ʽ__________��
(3)Ϊ��֤ʵ�龫ȷ�ȣ�����D��E��Ҫ�ֱ�ϴ�ӣ�����ϴ��Һת�ƻ�ĸҺ�У���
�жϳ���D�Ѿ�ϴ�Ӹɾ��ķ�����__________��
(4)��KMnO![]() ����Һ�ζ�C��Һʱ�������ķ�ӦΪ��
����Һ�ζ�C��Һʱ�������ķ�ӦΪ��
![]() ��
��
�ֽ���ҺCϡ����500 mL����ȡ���е�25.00 mL��Һ���������ữ����0��1000
mol��L-1��KMnO4����Һ�ζ����յ�ʱ����KMnO4��Һ10.00 mL��
�ٴ˲�������������Ҫ�õ�������Щ����(��д���)________��
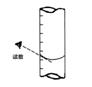
�ڴﵽ�ζ��յ�ʱ����Һ����ɫ�仯��________��
�۵ζ����յ㣬���ú�����ͼ��ȡKMnO4����Һ�Ŀ̶����ݣ���ⶨ�ĸ�Ԫ�غ�����________ (�ƫ�ߡ�����ƫ�͡�������Ӱ�족)��
(5)ԭ��Ҷ�и�Ԫ�ص���������Ϊ________��
(6)����ͨ��������ҺA����֤��Ʒ�Ʋ�Ҷ���Ƿ�����Ԫ�أ������Լ���ʵ��������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��12�֣�
A.ԭ������ͬ������������ͬ�ķ��ӣ�����Ϊ�ȵ����塣
��.��֪A��B��C��D��E���ַ�������ԭ�ӵ���Ŀ����Ϊ1��2��3��6��6���Ҷ�����18�����ӣ���֪B��C��D��������Ԫ�ص�ԭ����ɣ���D����������ԭ�Ӹ�����Ϊ1 :2��
��ش�
��1�����A���ӵ�ԭ�ӵ�Ԫ�ط����� ����֪E���ж����л��E���ۡ��е��CH4���ۡ��е�ߣ�����Ҫԭ����____________________________________��
��2��C������ṹ�� ____ �Σ��÷������� ���ӣ�����ԡ��Ǽ��ԡ�����
��3������пɳ���������������D��Ϊȼ�Ϸ�Ӧ���ɵ�����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ_______ __________________ ��������Ҫд��Ӧ������
��.CO��N2��Ϊ�ȵ����塣
��4��CO���ܼ��ܴ���N2���ܼ��ܣ���CO��N2���ײμӻ�ѧ��Ӧ��
�����±����ݣ�˵��CO��N2���õ�ԭ����____________________________________��
|
|
| A��B | A=B | A��B |
| CO | ���ܣ�kJ/mol�� | 357.7 | 798.9 | 1071.9 |
| ���ܲ�ֵkJ/mol�� | 441.2 273 | |||
| N2 | ���ܣ�kJ/mol�� | 154.8 | 418.4 | 941.7 |
| ���ܲ�ֵkJ/mol�� | 263.6 523.3 |
��5�����ǵķ����ж�����___________���Ҽ���______________���м���
��6��Fe��Co��Ni�Ƚ�������CO��Ӧ��ԭ������Щ����ԭ�ӵĵ��Ӳ�ṹ�йء�
Niԭ�ӵļ۵����Ų�ʽΪ _____ ��Fe(CO)5�����³�Һ̬���۵�Ϊ
��20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe(CO)5�������� ____��������ͣ���Fe(CO)5������������__________ ��
B�����к��ж������������彡���ijɷ֣��ݲⶨ��Ҷ�к���450�����ϵ��л��ɷ���15�����ϵ�Ԫ�ء�ij��ѧ�о�С����̽����Ҷ�и�Ԫ�صĺ����������̽��ʵ�鷽�����£�����֪��Ҷ�е�������Ԫ�ضԸ����ӵIJⶨ��Ӱ�죩
����1����ȡ500g����IJ�Ҷ������ͨ����У��������ʹ��Ҷ�һ��������в�ĥϸ�������ձ��У�Ȼ��200mL 1 mol��L-1���������н��衢���ˡ�ϴ�ӡ�
����2������1������Һ����μ���ϡ����������Һ��������Һ��pH��6��7���ң�ʹ������Ԫ���������������ʽ��ȫ�������ټ������30 min������7.95g��ˮ̼���ƣ���ֽ��裬��������ȫ���ˣ�ϴ�ӣ����˺�õ���Һ�ͳ�����
����3��������2���õ���Һϡ����500 mL��ȡ���е�20.00 mL��Һ�Լ�����ָʾ������0.100mol��L-1��HCl����Һ�ζ����յ�ʱ������������Ϊ20.00mL����������
��ش��������⣺
���� 1�У�ʹ��Ҷ�һ�ʱ��Ҫ�õ����Ǽܡ������ǡ��ƾ���ơ� ___ �� ____ ��������
����2�У������Լ� _______ (д�Լ�����)������pH����Ϊ���㣻�жϳ����Ѿ�ϴ���ķ����� ��
����3�У��ζ������У��۾�Ӧע�� ____________________�����ζ���20 mL��Һ�к�CO32�������ʵ���Ϊ __ mol���Լ���ԭ500g��Ҷ�и����ӵ���������Ϊ _______ ������������£�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011������ʡ��������ѧУ����������ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
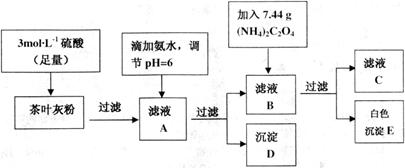
��Ҷ�к��ж������������彡�����л��ɷּ��ơ�����������Ԫ�أ�ij��ѧ�о���ѧϰС����Ʒ������ԲⶨijƷ�Ʋ�Ҷ�и�Ԫ�ص�����������������Ԫ�صĴ���(��֪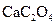 Ϊ��ɫ��������)������ȡ200g��Ҷ��Ʒ���յûҷۺ�������²�����
Ϊ��ɫ��������)������ȡ200g��Ҷ��Ʒ���յûҷۺ�������²�����
��ش������й����⣺
(1)����������ʾ��ijЩ�������ӵ�����������ȫ������pHΪ����
| ���� | 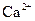 | 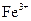 |
| ��ȫ����ʱ��pH | 13 | 4.1 |
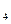 ����Һ�ζ�C��Һʱ�������ķ�ӦΪ��
����Һ�ζ�C��Һʱ�������ķ�ӦΪ��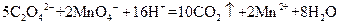 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ������ʡ����������ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��Ҷ�к��ж������������彡�����л��ɷּ��ơ�����������Ԫ�أ�ij��ѧ�о���ѧϰС����Ʒ������ԲⶨijƷ�Ʋ�Ҷ�и�Ԫ�ص�����������������Ԫ�صĴ���(��֪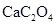 Ϊ��ɫ��������)������ȡ200g��Ҷ��Ʒ���յûҷۺ�������²�����
Ϊ��ɫ��������)������ȡ200g��Ҷ��Ʒ���յûҷۺ�������²�����
��ش������й����⣺
(1)����������ʾ��ijЩ�������ӵ�����������ȫ������pHΪ����
|
���� |
|
|
|
��ȫ����ʱ��pH |
13 |
4.1 |
ʵ��ǰҪ�Ƚ���Ҷ��Ʒ�������ճɻҷۣ�����ҪĿ����__________��
(2)д������ҺA������D�����ӷ�Ӧ����ʽ__________��
(3)Ϊ��֤ʵ�龫ȷ�ȣ�����D��E��Ҫ�ֱ�ϴ�ӣ�����ϴ��Һת�ƻ�ĸҺ�У���
�жϳ���D�Ѿ�ϴ�Ӹɾ��ķ�����__________��
(4)��KMnO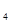 ����Һ�ζ�C��Һʱ�������ķ�ӦΪ��
����Һ�ζ�C��Һʱ�������ķ�ӦΪ��
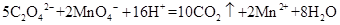 ��
��
�ֽ���ҺCϡ����500 mL����ȡ���е�25.00 mL��Һ���������ữ����0��1000
mol��L-1��KMnO4����Һ�ζ����յ�ʱ����KMnO4��Һ10.00 mL��
�ٴ˲�������������Ҫ�õ�������Щ����(��д���)________��

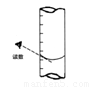
�ڴﵽ�ζ��յ�ʱ����Һ����ɫ�仯��________��
�۵ζ����յ㣬���ú�����ͼ��ȡKMnO4����Һ�Ŀ̶����ݣ���ⶨ�ĸ�Ԫ�غ�����________ (�ƫ�ߡ�����ƫ�͡�������Ӱ�족)��
(5)ԭ��Ҷ�и�Ԫ�ص���������Ϊ________��
(6)����ͨ��������ҺA����֤��Ʒ�Ʋ�Ҷ���Ƿ�����Ԫ�أ������Լ���ʵ��������________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com