
��ϸ��������һ����Ҫ�Ĺ����մ�ԭ�ϡ�
��1��ʵ���ҳ���NH4Al��SO4��2��NH4HCO3Ϊԭ�ϣ���һ���������ȷ�Ӧ���ɳ���NH4AlO��OH��HCO3���ó������·ֽ⼴�ó�ϸAl2O3��NH4AlO��OH��HCO3�ȷֽ�Ļ�ѧ��Ӧ����ʽΪ_________________________��
��2��NH4Al��SO4��2��12H2O����Է�������Ϊ453��������100 mL pHΪ2��Ũ��ԼΪ0.1 mol��L-1��NH4Al��SO4��2��Һ�����ƹ���Ϊ��
����������ƽ����NH4Al��SO4��2��12H2O����______________________g��
�ڽ��������������ձ��У�_________________________��
��3����0.1 mol��L-1 NH4Al��SO4��2��Һ�У�������̬��Ũ�ȣ���Al3+�ƣ��Ķ�����lgc������ҺpH�仯�Ĺ�ϵ����ͼ��

����NaOH��Һ���ڣ�2������ҺpH��7���ù����з�����Ӧ�����ӷ���ʽ��___________��
����������Ŀ�ͼ�У�����0.01 mol��L-1 NH4Al��SO4��2��Һ��������̬��Ũ�ȵĶ���lgc����ҺpH�仯�Ĺ�ϵͼ�������б�Ҫ��ע��
���𰸡�
��1��2NH4AlO��OH��HCO3 Al2O3+2CO2��+2NH3��+3H2O��
Al2O3+2CO2��+2NH3��+3H2O��
��2����4.5 �ڼ�����ϡ�����ܽ⣬�ټ�ˮϡ����100 mL
��3����Al3++3OH-====Al(OH)3�� H++OH-====H2O 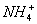 +OH-====NH3��H2O
+OH-====NH3��H2O
��
��������(2)��m��0.1 mol��L-1��0.1 L��453 g��mol-1��4.5 g
����������Һ��pHΪ2������������ҺʱҪ����������ϡ���ᡣ
��3����pH��7ʱ��Al��OH��3���ܽ⣬ֻ��һ�����ӷ�Ӧ������
��0.01 mol��L-1 NH4Al��SO4��2��Һ��lgc��-2��


 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪ij���������к���NaCl���ʣ�Ϊ�ⶨ�����д��������������������ͼ�е�װ�ý���ʵ�顣

��Ҫʵ�鲽�����£�
�ٰ�ͼ��װ�����������װ�õ�������
�ڽ�a g����������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ
�۳���ʢ�м�ʯ�ҵ�U�ܵ��������õ�b g
�ܴӷ�Һ©������6mol��L��1�����ᣬֱ�����ٲ�������ʱΪֹ
�ݴӵ���A����������һ�����Ŀ���
���ٴγ���ʢ�м�ʯ�ҵ�U�ܵ��������õ�c g
���ظ�����ݺ͢IJ�����ֱ��U�ܵ������������䣬Ϊd g
����պͻش����⣺
��1������������ƽ������Ʒʱ�������ƽ��ָ������ƫת��˵��
��2��װ���и����B��������
��3���������Һ©���е����ỻ��Ũ����ͬ�����ᣬ���ԵĽ�� (��ƫ�ߡ�ƫ�ͻ�)
��4������ݵ�Ŀ����
��5������ߵ�Ŀ����
��6���������д����������������ʽΪ
��7��������������ʵ�鷽���ⶨ�����д�������������������һ�ֲ�ͬ��ʵ�鷽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȩ��һ�ֻ���ԭ�ϡ�ijʵ��С����������װ�úϳ�����ȩ��

 �����ķ�Ӧ���£�
�����ķ�Ӧ���£�
CH3CH2CH2CH2OH CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
| �е�/��c | �ܶ�/(g��cm-3) | ˮ���ܽ��� | |
| ������ | 11.72 | 0.8109 | �� |
| ����ȩ | 75.7 | 0.8017 | �� |
ʵ�鲽�����£�
��6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�С���A�м���4.0g�������ͼ�����ʯ�����ȡ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����µ���֡�
������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�����2.0g��
�ش��������⣺
��1��ʵ���У��ܷ� Na2Cr2O7��Һ�ӵ�Ũ�����У�˵������
��
��2�������ʯ�������� �������Ⱥ���δ�ӷ�ʯ��Ӧ��ȡ����ȷ������ ��
��3������װ��ͼ�У�B������������ ��D������������ ��
��4����Һ©��ʹ��ǰ������еIJ����� ������ȷ�𰸱�ţ���
a.��ʪ b.���� c.��© d.�궨
��5��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ��ˮ�� �㣨��ϡ����¡�
��6����Ӧ�¶�Ӧ������90��95�棬��ԭ���� ��
��7����ʵ���У�����ȩ�IJ���Ϊ %��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������һ����Ҫ�Ļ�����Ʒ��ʵ������������������ˮ��Һ�Ʊ������װ����ͼ14��ʾ�����ȡ�����������̶�װ�þ�����ȥ��ʵ��������£�

�ٽ�һ�����ĵ���ˮ��Һ��������ƿ��
�ڿ��Ʒ�ӦҺ�¶���55��60�������£��߽�������μ�һ�����������������Ļ��ᣨ65%HNO3��98%H2SO4��������Ϊ2��1.5����Һ
�۷�Ӧ3h���ң���ȴ�����˺����ؽᾧ�ò��ᾧ�塣
������������ˮ��Һ�����пɷ������з�Ӧ��
C6H12O6��12HNO3��3H2C2O4��9NO2����3NO����9H2O
C6H12O6��8HNO3��6CO2��8NO����10H2O
3H2C2O4��2HNO3��6CO2��2 NO����4H2O
NO����4H2O
(1)��������Ƿ�ˮ����ȫ�����õ��Լ�Ϊ ��
(2)ʵ����������μӹ��죬�����²�������½�����ԭ���� ��
(3)װ��C����β�����գ���β����n(NO2):n(NO)��1��1ʱ��������NaOH��Һ�ܽ�NOxȫ�����գ�ԭ���� ���û�ѧ����ʽ��ʾ��
(4)����NaOH��Һ����β����Ƚϣ����õ���ˮ��Һ����β�������š�ȱ���� ��
(5)�����ؽᾧ�ļ�ѹ���˲����У����ձ����������⣬������ʹ�����ڹ����β��ϵ������� ��
������������һ����ʵ�黯ѧ���Ļ��������⣬ͨ�������Ʊ�������ѧ���Ի�ѧԭ������ѧʵ�鷽�������ճ̶ȣ���ʵ�鷽���ķ������ۺ����û�ѧ֪ʶ�����ѧʵ���еľ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ�
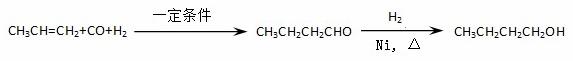
CO���Ʊ�ԭ����HCOOH CO��+H2O������Ƴ�ԭ�������Ʊ�װ�ã�����ͼ��
CO��+H2O������Ƴ�ԭ�������Ʊ�װ�ã�����ͼ��

����д���пհף�
��1��ʵ��������п����ϡ���ᡢϡ���ᡢŨ���ᡢ2-����������ѡ����ʵ��Լ��Ʊ���������ϩ��д����ѧ����ʽ�� �� ��
��2����������װ���Ʊ����﴿����CO��װ���� a��b�����÷ֱ��� �� ��
a��b�����÷ֱ��� �� ��
C��d�г�װ���Լ��ֱ��� �� ����������װ���Ʊ�H2�� ���巢��װ���б���IJ������������� �������߿��ڻ����ռ�����H2��װ��ͼ��
��3���Ʊ�ϩʱ������������SO2��CO2��ˮ��������С���������Լ��������������壬�������ͨ���Լ���˳����________________������ţ�
�ٱ���Na2SO3��Һ ������KMnO4��Һ ��ʯ��ˮ
����ˮCuSO4 ��Ʒ����Һ
��4���ϳ�����ȩ�ķ�ӦΪ������ȵĿ��淴Ӧ��Ϊ����Ӧ���ʺ����ԭ������ת���ʣ�����ΪӦ�ò��õ����˷�Ӧ������______________��
a. ���¡���ѹ������ b. �ʵ����¶ȡ���ѹ������
c. ���¡���ѹ������ d. �ʵ����¶ȡ���ѹ������
��5������ȩ����������õ�����������ȩ��1��������Ʒ��Ϊ����1����������С���������֪����R��CHO+NaHSO3�����ͣ���RCH(OH)SO3Na�����ڷе㣺����34�棬1������118�棬����Ƴ������ᴿ·�ߣ�
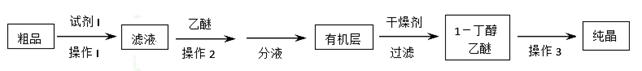
�Լ�1Ϊ_________������1Ϊ________������2Ϊ_______������3Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���ҽ�9 g���۸�һ�����Ľ����������ĩ����γ����ȼ����������ȷ�Ӧ֮�����ù����к���������18 g������������ĩ������( )
A.Fe2O3��MnO2 B.MnO2��V2O5
C.Cr2O3��V2O5 D.Fe3O4��FeO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1 mol����������2 mol̼�����ƹ����Ϻ����ܱ������м��ȳ�ַ�Ӧ���ų��������ʺ���ȴ�������Ĺ���������
A.Na2CO3 B.Na2O2 Na2CO3
C.NaOH Na2CO3 D.Na2O2 NaOH Na2CO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�������£�������������Ӧ��������1.5g ����ˮ��������Һǡ���ܱ�80mLŨ��Ϊ0.50mol/L��HCl��Һ�кͣ����������ijɷ���
A.Na2O B.Na2O2
C.Na2O��Na2O2 D.Na2O2��NaO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ȷ���ǣ� ����
A��������Ԫ�ص����ӣ���һ������������
B����������ԭ��Ӧ�У��ǽ�������һ����������
C��ijԪ�شӻ���̬��Ϊ����̬ʱ����Ԫ��һ������ԭ
D�����������ӱ���ԭһ���õ���������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com