
ijŹµŃ銔×éÅäÖĘ0.10mol/L NaOHČÜŅŗ²¢½ųŠŠÓŠ¹ŲŠŌÖŹŹµŃ飬»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©ČōŹµŃéÖŠ“óŌ¼ŅŖŹ¹ÓĆ475mL NaOHČÜŅŗ£¬ÖĮÉŁŠčŅŖ³ĘĮæNaOH¹ĢĢå g”£
£Ø2£©“ÓĻĀĶ¼ÖŠŃ”Ōń³ĘĮæNaOH¹ĢĢåĖłŠčŅŖµÄŅĒĘ÷ŹĒ£ØĢī×ÖÄø£© ”£
| Ćū³Ę | ĶŠÅĢĢģĘ½ £Ø“ųķĄĀė£© | Š”ÉÕ± | ŪįŪöĒÆ | ²£Į§°ō | Ņ©³× | ĮæĶ² |
| ŅĒĘ÷ | | | | | | |
| ŠņŗÅ | a | b | c | d | e | f |
£Ø3£©ĻĀĮŠĒéæö»įŹ¹ĖłÅäČÜŅŗÅضČĘ«µĶµÄŹĒ£ØĢīŠņŗÅ£©
¢Ł³ĘĮæŹ±£¬×óÅĢøߣ¬ÓŅÅĢµĶ
¢Ś¹ĢĢåČܽāŗóĪ“ĄäČ“µ½ŹŅĪĀ¾ĶÖ±½Ó×ŖŅʵ½ČŻĮæĘæÖŠ
¢ŪČÜŅŗ×ŖŅʵ½ČŻĮæĘæŗó£¬Ī“½ųŠŠĻ“µÓ²Ł×÷
¢Ü×ŖŅĘČÜŅŗĒ°ČŻĮæĘæÄŚÓŠÉŁĮæÕōĮóĖ®
¢Ż¶ØČŻŹ±£¬ŃöŹÓČŻĮæĘæµÄæĢ¶ČĻß
¢Ž¶ØČŻŗóŅ”ŌČ£¬·¢ĻÖŅŗĆę½µµĶ£¬ÓÖ²¹¼ÓÉŁĮæĖ®£¬ÖŲŠĀ“ļµ½æĢ¶ČŹµŃéŹŅÓūÅäÖĘ
£Ø4£©ĻņVmLÉĻŹöÅØ¶ČµÄNaOHČÜŅŗÖŠ£¬ĶØČėŅ»¶ØĮæµÄCO2ŗó£¬Č»ŗóĻņøĆČÜŅŗÖŠÖšµĪ¼ÓČė1mol L-1µÄŃĪĖį£¬Ėł¼ÓČėŃĪĖįČÜŅŗµÄĢå»żÓė²śÉśCO2µÄĢå»ż¹ŲĻµČēĻĀĶ¼ĖłŹ¾£ŗ
L-1µÄŃĪĖį£¬Ėł¼ÓČėŃĪĖįČÜŅŗµÄĢå»żÓė²śÉśCO2µÄĢå»ż¹ŲĻµČēĻĀĶ¼ĖłŹ¾£ŗ
 |
¢Łµ±¼ÓČė35.0mLŃĪĖįČÜŅŗŹ±£¬²śÉś±ź×¼×“æöĻĀ¶žŃõ»ÆĢ¼µÄĢå»żĪŖ__________mL£»
¢Ś¼ĘĖćĖłČ”ĒāŃõ»ÆÄĘČÜŅŗµÄĢå»żV=__________mL”£
£Ø5£©Č”ÉĻŹöĒāŃõ»ÆÄĘČÜŅŗ200mL£¬¼ÓČėŹŹĮæĀĮ·ŪŹ¹Ö®Ē”ŗĆĶźČ«·“Ó¦£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________________________________£¬Öš½„Ļņ·“Ó¦ŗóµÄČÜŅŗÖŠÖĮÉŁ¼ÓČė1.0 mol L-1µÄŃĪĖį________ mL²ÅÄÜŹ¹Éś³ÉµÄ³ĮµķĶźČ«Čܽā”£
L-1µÄŃĪĖį________ mL²ÅÄÜŹ¹Éś³ÉµÄ³ĮµķĶźČ«Čܽā”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
A. Ēæµē½āÖŹČÜŅŗµÄµ¼µēÄÜĮ¦Ņ»¶Ø±ČČõµē½āÖŹČÜŅŗµÄĒæ
B. °±ĘųŹĒČõµē½āÖŹ£¬ĶŹĒĒæµē½āÖŹ
C. Ńõ»ÆÄĘŹĒĒæµē½āÖŹ£¬“×ĖįŹĒČõµē½āÖŹ
D. ĮņĖįÄĘŹĒĒæµē½āÖŹ£¬ĮņĖį±µŹĒČõµē½āÖŹ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
X”¢Y”¢Z”¢MĖÄÖÖ½šŹō£¬ŅŃÖŖXæÉŅŌ“ÓYµÄŃĪČÜŅŗÖŠÖĆ»»³öY£ŗXŗĶZ×÷Ōµē³Ųµē¼«Ź±£¬ZĪŖÕż¼«£»YŗĶZµÄĄė×Ó¹²“ęÓŚµē½āŅŗÖŠ£¬YĄė×ÓĻČ·Åµē£»MµÄĄė×ÓµÄŃõ»ÆŠŌĒæÓŚYµÄĄė×Ó£®ŌņÕāĖÄÖÖ½šŹōµÄ»ī¶ÆŠŌÓÉĒæµ½ČõµÄĖ³ŠņĪŖ£Ø””””£©
| ”” | A£® | X£¾Y£¾Z£¾M | B£® | X£¾Z£¾M£¾Y | C£® | M£¾Z£¾X£¾Y | D£® | X£¾Z£¾Y£¾M |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĄė×Ó·½³ĢŹ½ŹéŠ“ÕżČ·µÄŹĒ
A. ŃĒĮņĖįÄĘČÜŅŗ¼ÓČėĻ”ĻõĖį£ŗSO32-£«2H+= SO2”ü£«H2O
B£®µāĖ®ÖŠĶØČė×ćĮæµÄSO2£ŗI2 +SO2+2H2O=2I£+SO42£+4H+
C£®NaHSO4ČÜŅŗÓėBa(OH)2ČÜŅŗ·“Ó¦ÖĮÖŠŠŌ£ŗH+£«SO42££«Ba2+£«OH£=BaSO4”ż£«H2O
D£®ŌŚĒæ¼īČÜŅŗÖŠ“ĪĀČĖįÄĘÓėFe(OH)3·“Ӧɜ³ÉNa2FeO4£ŗ
3ClO£+2Fe(OH)3=2FeO42£+3Cl£+H2O+4H+
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¶ŌÓŚ4”ꏱ100mLĖ®ÖŠČܽāĮĖ22.4 L HClĘųĢå(±ź×¼×“æöĻĀ²āµĆ)ŗóŠĪ³ÉµÄČÜŅŗ£¬ĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ
A£®øĆČÜŅŗĪļÖŹµÄĮæÅضČĪŖ10moL£ÆL
B£®ĖłµĆµÄČÜŅŗµÄĢå»żĪŖ22£®5L
C£®øł¾ŻĢāøÉŹż¾Ż£¬øĆČÜŅŗĪļÖŹµÄĮæÅضČĪŽ·ØĒóµĆ
D£®øĆČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżŅņČÜŅŗµÄĆܶČĪ“ÖŖ¶ųĪŽ·ØĒóµĆ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆѧÓėÉś»ī”¢Éś²śĆÜĒŠĻą¹Ų£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A£®Ģ¼Ėį±µ”¢Ģ¼ĖįĒāÄĘ”¢ĒāŃõ»ÆĀĮ¾łæÉ×÷ĪŖæ¹ĖįŅ©ĪļŹ¹ÓĆ
B£®ČĖĢåÄŚµÄµ°°×ÖŹ²»¶Ļ·Ö½ā£¬×īÖÕÉś³ÉĖ®ŗĶ¶žŃõ»ÆĢ¼ÅųöĢåĶā
C£®¹¤ŅµÉś²ś²£Į§ŗĶĖ®Äą£¬¾łŠčŅŖÓĆ“æ¼īĪŖŌĮĻ
D£®”°µŲ¹µÓĶ”±µÄÖ÷ŅŖ³É·ÖŹĒÓĶÖ¬£¬Ęä×é³ÉÓėĘūÓĶ”¢ĆŗÓĶ²»ĻąĶ¬
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅųÖŹĘ÷ĆóČÕ¾Ć±ķĆę»įÖš½„±äŗŚ£¬ÕāŹĒÉś³ÉĮĖAg2SµÄŌµ¹Ź”£øł¾Żµē»ÆѧŌĄķæɽųŠŠČēĻĀ“¦Ąķ£ŗŌŚĀĮÖŹČŻĘ÷ÖŠ¼ÓČėŹ³ŃĪČÜŅŗ£¬ŌŁ½«±äŗŚµÄŅųĘ÷ĀžČėøĆČÜŅŗÖŠ£¬Ņ»¶ĪŹ±¼äŗó·¢ĻÖŗŚÉ«»įĶŹČ„”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A£®“¦Ąķ¹ż³ĢÖŠŅųĘ÷Ņ»Ö±±£³ÖŗćÖŲ
B£®ŅųĘ÷ĪŖÕż¼«£¬Ag2S±»»¹ŌÉś³Éµ„ÖŹŅų
C£®øĆ¹ż³ĢÖŠ×Ü·“Ó¦ĪŖ2Al+3Ag2S=6Ag+A12S3
D£®ŗŚÉ«ĶŹČ„µÄŌŅņŹĒŗŚÉ«Ag2S×Ŗ»ÆĪŖ°×É«AgC1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĶ¼ŹĒij»ÆѧŠĖȤŠ”×éÉč¼ĘµÄĄūÓƵē×ÓĄ¬»ų(ŗ¬70£„Cu”¢25£„Al”¢4£„Fe¼°ÉŁĮæAu”¢Pt)ÖʱøĮņĖįĶŗĶĮņĖįĀĮ¾§ĢåµÄĀ·Ļߣŗ
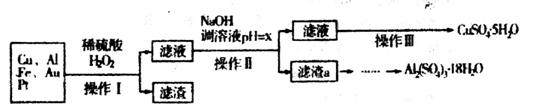
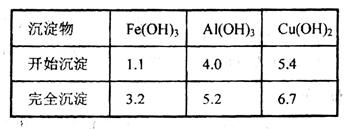
ŅŃÖŖĻĀĮŠŠÅĻ¢£ŗCuæÉÓėĻ”ĮņĖįŗĶH2O2µÄ»ģŗĻŅŗ·“Ӧɜ³ÉĮņĖįĶ£»Ģś”¢ĀĮ”¢ĶµČĄė×ÓŅŌĒāŃõ»ÆĪļŠĪŹ½³ĮµķŹ±ČÜŅŗµÄpHČēĻĀ±ķ£ŗ
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öCuÓėĻ”ĮņĖįŗĶH2O2µÄ»ģŗĻŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½______________”£
£Ø2£©ŌŚ²Ł×÷IIÖŠ£¬xµÄȔֵ·¶Ī§ŹĒ___________________”£
£Ø3£©ŌŚ²Ł×÷IIIÖŠ£¬Õō·¢ÅØĖõŠčŅŖµÄ²£Į§ŅĒĘ÷ÓŠ______________________________________”£
£Ø4£©ÓÉĀĖŌüaÖĘČ”Al2£ØSO4£©3•18H2O£¬Ģ½¾æŠ”×éÉč¼ĘĮĖČżÖÖ·½°ø£ŗ
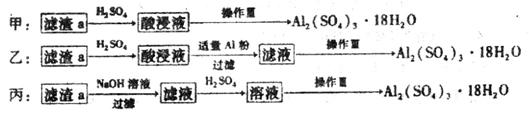
×ŪŗĻæ¼ĀĒÉĻŹöČżÖÖ·½°ø£¬×ī¾ßæÉŠŠŠŌµÄŹĒ___________£ØĢīŠņŗÅ£©”£
£Ø5£©ĪŖ²ā¶ØCuSO4•5H2O¾§ĢåµÄ“æ¶Č£¬½ųŠŠĻĀĮŠŹµŃé£ŗČ”a g ŹŌŃłÅä³É100mLČÜŅŗ£¬Ćæ“ĪČ”20.00mL£¬Ļū³żøÉČÅĄė×Óŗó£¬ÓĆb mol/LEDTA(Na2H2Y)±ź×¼ČÜŅŗµĪ¶ØĘäÖŠµÄCu2£«£ØĄė×Ó·½³ĢŹ½ĪŖCu2++H2Y2-£½CuY2-+2H+£©£¬µĪ¶ØÖĮÖÕµć£¬Ę½¾łĻūŗÄEDTAČÜŅŗ12.00mL£¬CuSO4•5H2O¾§ĢåµÄ“æ¶ČŹĒ_________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
±ź×¼×“æöĻĀ£¬COŗĶCO2µÄ»ģŗĻĘųĢåµÄÖŹĮæŹĒ9.6g£¬Ģå»żŹĒ6.72L£¬ŌņCO2ŌŚ»ģŗĻĪļÖŠµÄÖŹĮæ·ÖŹżŌ¼ĪŖ£Ø £©
A£®25% B£®3£“% C£®75% D£®50%
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com