
��ϩ��������������������Ʊ����ϡ���֬�ȸ߾������Ҫ�м��壬��������·�ߺϳɣ�
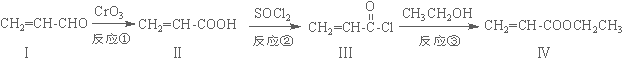 ��1����������ķ���ʽΪ ��1 mol���������ȫȼ������O2Ϊ mol��
��1����������ķ���ʽΪ ��1 mol���������ȫȼ������O2Ϊ mol��
��2�����������ʹ��ˮ��ɫ���䷴Ӧ����ʽΪ ��
��3����Ӧ������ ��Ӧ�������������ɻ������������ʽΪC3H6O���������õ������������ķ�Ӧ����ʽΪ ��
��4����������ǻ��������ͬ���칹�壬������̼̼˫��������NaHCO3��Һ��Ӧ�ų����壬��˴Ź����������֮��Ϊ1��1��6��������Ľṹ��ʽΪ ��
��5��һ�������£�������  Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ ��
Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ ��
��֪ʶ�㡿�л���Ľṹ������ L2 L4 L7
���𰸽�����(1)C5H8O2 �� 6��
(2)CH2=CH-COOH+Br2��CH2Br-CHBr-COOH
(3)ȡ�� 2CH2=CH-CH2OH+O2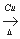 2CH2=CH-CHO+2H2O
2CH2=CH-CHO+2H2O
(4)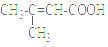 (5)
(5)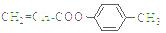
��������1�����������һ��������5��̼ԭ�ӡ�8����ԭ�ӡ�2����ԭ�ӣ�����ʽΪC5H8O2��1 mol���������ȫȼ������O2Ϊ����5+2-1��=6mol��
��2���������ķ��ӽṹ�к�̼̼˫��������ˮ�����ӳɷ�Ӧ���䷴Ӧ����ʽ��CH2=CH-COOH+Br2��CH2Br-CHBr-COOH
��3�����������ӽṹ�е��ǻ�����ԭ��ȡ���������������ɻ������������ʽΪC3H6O���������õ��������Ϊ���࣬�ṹ��ʽ��CH2=CH-CH2OH����Ӧ����ʽΪ�� 2CH2=CH-CH2OH+O2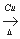 2CH2=CH-CHO+2H2O
2CH2=CH-CHO+2H2O
��4����������ǻ��������ͬ���칹�壬������NaHCO3��Һ��Ӧ�ų�����õ������Ȼ�����˴Ź����������֮��Ϊ1��1��6��������Ľṹ��ʽΪ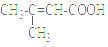
��ȷ�Ӧ�ۣ����ȡ����Ӧ���ص㼴�ɵõ�����Ľṹ��ʽ��
��˼·�㲦�����⿼�����л�������ż��ת�����Ƚϻ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ��������ġ��������䡱���̣�
(1)���еġ�������ָ________����
(2)����Ҫ�ɷֵķ���ʽ��________������ʽΪ____________________________________��
�ṹʽΪ________��
(3)�������ԭ�ӵĿռ�ֲ���________�ṹ��
(4)���ʺ��ڱ�ʾ���ӿռ乹�͵�ģ����______________________________________��
(5)����Ҫ�ɷ���Cl2�ڹ��������µķ�Ӧ����________(�Ӧ����)���������ɵ��л�����ֱ���__________��________��________��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��
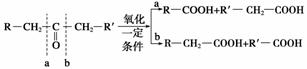
�ֽ�A��������ͼ��ʾ��Ӧ��
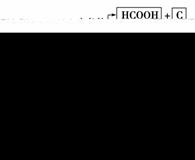
��֪��D����Է���������EС��B���ܷ���������Ӧ��Fʹ��ˮ��ɫ�������к�����
�Իش��������⣺
(1)д���������ʵĽṹ��ʽ��A________��C________��G________��
(2)д�����б仯�Ļ�ѧ����ʽ��
��A��B��________________________________________________________________________��
��A��H��________________________________________________________________________��
��E���ڱ�����(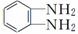 )��һ�������¾ۺ�����һ�ֺϳ���ά��________________________��
)��һ�������¾ۺ�����һ�ֺϳ���ά��________________________��
(3)��֪�л�������ͬһ̼ԭ���Ͻ������ǻ��Dz��ȶ��ģ����Զ���ˮ��
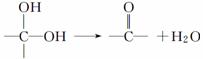
A���ܷ���������Ӧ���������������Ʒ�Ӧ�ų�H2��ͬ���칹����________�֡�д�������ں˴Ź����������������������ڼ���������ˮ��Ļ�ѧ����ʽ��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ��ȼ�ϵ���з����Ļ�ѧ��ӦΪ����������Һ�м״���������������ˮ�Ͷ�����̼���õ�ظ��������ķ�Ӧ��(����)
A��CH3OH(g)��O2(g)����H2O(l)��CO2(g)��2H��(aq)��2e��
B��O2(g)��4H��(aq)��4e������2H2O(l)
C��CH3OH(g)��H2O(l)����CO2(g)��6H��(aq)��6e��
D��O2(g)��2H2O(l)��4e������4OH��(aq)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ŵ�����ɰ�˾ƥ�֡�����Ϣʹ����ѧ��ƴ���Ʊ��Ľ�����ʹ����ҩ����ϳɷ�Ӧʽ (��Ӧ������ȥ)����:

���������������
A. FeCl3��Һ������˾ƥ�ֺ�����Ϣʹ
B.1 mol��ŵ����������3 mol NaOH
C.�����£���ŵ����ˮ�е��ܽ��С������Ϣʹ
D.�밢˾ƥ�ֻ�Ϊͬ���칹�壬��1 mol��������NaHCO3��Һ��Ӧ����2mo1CO2�ĽṹΪ10��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͭ������í������������ʴ������˵���У�����ȷ����(����)

A�������缫��ӦʽΪ��2H����2e������H2��
B���˹����л��漰����Ӧ��4Fe(OH)2��2H2O��O2===4Fe(OH)3
C���˹�����ͭ��������ʴ
D���˹����е��Ӵ�Fe����Cu
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ�����������Ʋ�ͬ���������������������У��ټ��뺬��������̪��NaCl����֬����Һ����ȴ���γ�����(�����������ڿ����ƶ�)������������ȷ����(����)
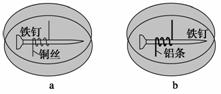
A��a�������������ֺ�ɫ B��b�������Ϸ�����ԭ��Ӧ
C��a��ͭ˿�Ϸ���������Ӧ D��b���������������ݲ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��95��ʱ��KW=1.0��10��12.�ڸ��¶���,���0.1mol��L��1Na2A��ҺPH=6,������˵����ȷ����
A��H2A��ˮ��Һ�еĵ��뷽��ʽΪ��H2A 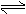 H++HA-��HA��
H++HA-��HA��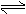 H++A2-
H++A2-
B����NH4��2A��Һ�д�������Ũ�ȹ�ϵ��c��NH4+��>c��A2-��>c(H+��>c��OH����
C��0.0lmol��L-l��H2A��ҺpH=2
D���������Ũ�ȵ�������H2A��Һ�ֱ���5.6g Zn��Ӧ��H2A��Һ������H2��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com