
��ҵ�����õ�����ࣨ��Ҫ����Fe2O3��CuO��Cr2O3�������������ʣ�����ͭ���Ƚ����������������£�
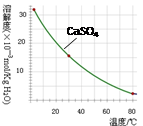
��֪�������ʳ�����pH��CaSO4���ܽ���������£�
| | Fe3+ | Cu2+ | Cr3+ |
| ��ʼ����pH | 2��1 | 4��7 | 4��3 |
| ��ȫ����pH | 3��2 | 6��7 | a |
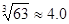 ��
�� ��1��CuSO4 (3��)
��2����3��2 (2��) �ڳ��ȹ��� (2��)
��3��2H2O + HSO3�� +2Cu2+=Cu2O��+SO42�� +5H+(3��)�� ����SO2��Ⱦ����(3��)
��4��4��0��10-9mol/L (3��)
���������������1��Fe2O3��CuO��Cr2O3�����ᷴӦ����Fe2(SO4)3��CuSO4��Cr2(SO4)3�������ڽ��������г�������Fe2(SO4)3��Cr2(SO4)3��,��Ҫ����CuSO4��
��2���ٳ��������������ӳ�����ȫ����ͭ����������Ӳ��ܳ����������ӳ�����ȫ��pH��3��2���Ե�����ҺpH��3��2��80��ʱCaSO4���ܽ���Ѿ���С����ʱ�Թ�����ʽ���ڣ��������ȹ��ˣ��¶��½���CaSO4���ܽ�����������»ص���Һ�У��ﲻ������Ŀ�ģ����Խ���Һ���ȵ�80�棬Ҫ���ȹ��ˣ��Գ�ȥCaSO4
��3��CuԪ����Cu2+��ʽ��������Һ�У�����NaHSO3���߷���������ԭ��Ӧ������Cu2O���壬HSO3����������SO42�����������ӷ���ʽΪ2H2O+HSO3�� +2Cu2+=Cu2O��+SO42��+5H+����NaHSO3��������ò�����Cu2+����ԭ�����Һ������ǿ����H+���������NaHSO3��Ӧ����SO2����Ⱦ������
��4��Cr3+��ȫ����ʱC(Cr3+)��1��10��5 mol?L-1,��Ksp[Cr(OH)3]= C(Cr3+)��C(OH��)3=6��3��10-31��C(OH��)3= 6��3��10-31/ C(Cr3+)��6��3��10-26,����C(OH��) �� ��10-9=4��0��10-9��
��10-9=4��0��10-9��
���㣺����Թ�ҵ���̵ķ�������Ӧ���pH���жϣ�Ksp���йؼ��㣬���ӷ���ʽ����д


 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��һƿ��ɫ���壬���ܺ���HCl��H2S��CO2��HBr��SO2�е�һ�ֻ��֣�����ͨ����ˮ�е���ɫ����Һ������Һ�ֳ����ݣ���һ���м��������ữ��BaCl2��Һ�����ְ�ɫ��������һ���м��������ữ��AgNO3��Һ��Ҳ�а�ɫ���������½�����ȷ���ǡ����� ��
��ԭ�����п϶���SO2����ԭ�����п�����SO2����ԭ�����п϶�û��H2S��HBr���ܲ��ܿ϶�ԭ�������Ƿ���HCl����ԭ�����п϶���CO2����ԭ�����п϶���HCl.
| A���٢ۢ� | B���ڢ� | C���٢ۢ� | D���٢ۢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ī���Ρ����������(NH4)2SO4��FeSO4��6H2O����һ����Ҫ��ѧ�Լ���ʵ�����ú����۵���Ƭ����ȡĪ���Σ��������£�
��ش��������⣺
��1������10%Na2CO3��Һ��ԭ����___________________�������ӷ���ʽ��ʾ����
��2��A���ʿ���Ϊ________(����)��
a��CuCl2 b��CuO c��Cu(NO3)2 d��CuSO4
��3��B���ʵijɷ�Ϊ_________________��
��4�� �������������pHΪ 1��2��Ŀ����_____________________________��
�����������_______________________________________________��
��5������ˮ�Ҵ�ϴ�ӳ�ʪ����������茶�����Ϊ�˳�ȥ������������ˮ�֣����ü��Ⱥ�ɵ�ԭ����_________________________________________��
��6���������þ����к���Fe2����NH ��SO
��SO ���ӵ�ʵ�鷽����ȷ����________(����)��
���ӵ�ʵ�鷽����ȷ����________(����)��
a��ȡ������Ʒ���Թܣ���ˮ�ܽ⣬ͨ������Cl2���ټ�KSCN��Һ���۲�����
b��ȡ������Ʒ���Թܣ���ˮ�ܽ⣬��������KMnO4��Һ���۲�����
c��ȡ������Ʒ���Թܣ���ˮ�ܽ⣬����ŨNaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��۲���ֽ��ɫ�ı仯
d��ȡ������Ʒ���Թܣ���ˮ�ܽ⣬����������ټ���BaCl2��Һ���۲�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
V2O5�ǽӴ������������Ҫ��������ҵ������V2O5�Ĺ����������£���ش��������⣺
��1����������÷����ijɷ��� (д��ѧʽ)������NaOH��Һ��Ӧ�����ӷ�Ӧ����ʽΪ ��
��2������ڡ��۵ı仯���̿ɼ�Ϊ(��ʽR��ʾVO2+��HA��ʾ�л���ȡ��)��
R2(SO4)n(ˮ��)+2nHA(�л���) 2RAn(�л���)+nH2SO4(ˮ��)��
2RAn(�л���)+nH2SO4(ˮ��)��
��ʵ�����в���ڡ���ʹ�õ���Ҫ������ ��
������ȡʱ��������������ԭ���� ��
��3���������X�Լ�Ϊ ������ܵ�Ŀ���� ������ݵ����ӷ���ʽΪ ��
��4���ù��������У�����ѭ�����õ������� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ɸ������������ǿ�����ȶ��Ըߵ��������ܣ�ʹ�÷���ɸ��ù㷺Ӧ�ã�ij���ͺŷ���ɸ�Ĺ�ҵ�������̿ɱ�ʾ���£�
�ڵμӰ�ˮ����pH=9�Ĺ����У���pH���Ʋ���������Al(OH)3���ɣ�����������������Ԫ�ء���Ԫ�ؾ�û����ʧ����ԭ��������Ϊ10%��
��1������ɸ��ֱ��Ϊ4A(1A=10��10m)��Ϊ4A�ͷ���ɸ����Na+��Ca2+ȡ��ʱ���Ƶ�5A�ͷ���ɸ����Na+��K+ȡ��ʱ���Ƶ�6A�ͷ���ɸ��Ҫ��Ч����������(����ֱ��Ϊ4��65A)���춡��(����ֱ��Ϊ5��6A)Ӧ��ѡ��_______�ͷ���ɸ��
��2��Al2(SO4)3��Һ��Na2SiO3��Һ��Ӧ���ɽ�������ӷ���ʽΪ________________________
��3��������������������Һ�ﺬ�е����ӳ�H+��OH���⣬��Ҫ���� �����ӡ��������н��������ӵIJ��������� ��
��4����NH3?H2O����pH���ȵ�90�沢���ȹ��˵�ԭ������� ��
��5�����������������÷���ɸ�Ļ�ѧʽΪ ��������������ʽ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�ϳ��ø�������Ч�ɷ�ΪFeO��Cr2O3����Ҫ����ΪSiO2��Al2O3��Ϊԭ�������ظ���أ�K2Cr2O7����ʵ����ģ�ҵ���ø��������ظ���ص���Ҫ������������ͼ���漰����Ҫ��Ӧ�ǣ�6FeO��Cr2O3��24NaOH��7KClO3=12Na2CrO4��3Fe2O3��7KCl��12H2O���Իش��������⣺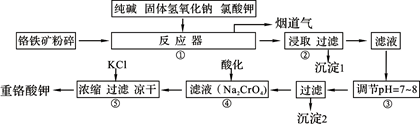
��1��������Һ���������ӵļ��鷽���� ��
��2������۱�����������Ϊ�������ӷ��ţ� ��
��3���ڷ�Ӧ�����У����������봿�Ӧ�Ļ�ѧ����ʽΪ�� ��
��4���̵����е�CO2����H2�ϳɼ״���CH3OH��H2��ȼ���ȷֱ�Ϊ����H=��725.5 kJ/mol����H=��285.8 kJ/mol��д����ҵ����CO2��H2�ϳ�CH3OH���Ȼ�ѧ����ʽ�� ��
��5��2011�����������ĸ���Ⱦ�¼���˵��������������ˮ���������ŷŶ��������滷���м����Σ������ⷨ�Ǵ�������Ⱦ��һ�ַ�������������������ʯī��������⺬Cr2O72-�����Է�ˮ��һ��ʱ������Fe(OH)3��Cr(OH)3������
��д����ⷨ������ˮ���ܷ�Ӧ�����ӷ���ʽ ��
����֪Cr(OH)3��Ksp=6.3��10�C31�����ر�ˮ�����������ֵ��0.1 mg/L��Ҫʹ��Һ��c(Cr3+)�������ϵر�ˮ��ֵ���������Һ��c(OH-)�� mol/L��ֻд�������ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ǧ�㷺Ӧ�����������ء�����пұ�������е�Ǧ������������Ǧ���������£�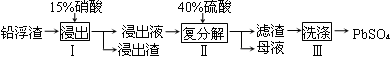
��֪Ǧ��������Ҫ�ɷ���PbO��Pb������������Ag��Zn��CaO��������������������ʡ�25��ʱ��Ksp(CaSO4)��4.9��10��5��Ksp(PbSO4)��1.6��10��8��
��1����֪�������NO����������Һ�к���������������Pb2+���ֱ�д��PbO��Pb�μӷ�Ӧ�����ӷ���ʽ �� ��
��2�����������������������ʹPb����ʣ�࣬Ŀ���� ��
��3��ĸҺ��ѭ�������ڲ������������Ҫ�� (��һ�����ʻ�ѧʽ)����ĸҺ�в�����SO42�����࣬ѭ������ʱ���ܳ��ֵ������� ��
��4����ƷPbSO4������Pb(NO3)2��Һ���ϴ�ӣ�Ŀ���dz�ȥ ��
��5��Ǧ���صĵ��Һ�����ᣬ���������缫�ϳ�����PbSO4�ֱ�ת��ΪPbO2��Pb�����ʱ�����ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��ɫ����Һ����ȷ���Ƿ����������ӣ� Fe2����Mg2����Al3����Ba2����NO3-��SO42-��Cl����I����HCO3-��ȡ����Һ����ʵ�飺
| ʵ�鲽�� | ʵ������ |
| (1)ȡ��������Һ���Ӽ�����ɫʯ����Һ | ��Һ��� |
| (2)ȡ��������Һ���ȣ���CuƬ��ŨH2SO4������ | ����ɫ���������������������ɺ���ɫ |
| (3)ȡ��������Һ����BaCl2��Һ | �а�ɫ���� |
| (4)ȡ(3)���ϲ���Һ����AgNO3��Һ | �а�ɫ�������Ҳ�����ϡHNO3 |
| (5)ȡ��������Һ����NaOH��Һ | �а�ɫ������NaOH����ʱ���������ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���þ���þ��(����MgO��KCl��MgCl2��BaCl2��CaCl2��FeCl3������)����MgCl2�Ĺ�ҵ��������:
��֪:25 ��ʱ�й����ʵ��ܶȻ�����:
| ���� | CaCO3 | MgCO3 | BaCO3 | Mg(OH)2 | Fe(OH)3 |
| Ksp | 4.96��10-9 | 6.82��10-6 | 5.1��10-9 | 5.61��10-12 | 2.64��10-38 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com