
Ϊ�˲ⶨijþ���Ͻ�ijɷ֣�ȡ14.7 g�Ͻ���ȫ����500 mL 3 mol/L�������У��ټ���400 mL 8 mol/L������������Һ��ַ�Ӧ�����ֻ����һ�ֳ���������ڸúϽ�IJⶨ���̵�������ȷ����( )
A���Ͻ���þ����������Ϊ63.3%��Mg%<100%
B���úϽ��к���������������Ϊ5.4 g
C���ڲ������������Һ��һ������0.2 mol NaAlO2
D���ڲ������������Һ����1.5 mol Na2SO4
AD
��������
���������������ü���ֵ����������ǡ����ȫ��Ӧ����������Ҳǡ����ȫ��Ӧ�����ʱ���ĺ���Ϊ���ֵ��������ת��Ϊƫ�������þ����ת��Ϊ������þ��������ʱ��Һ������ΪNa2SO4��NaAlO2������������غ���n��Na2SO4��=n��H2SO4��=0.5L�� 3 mol/L=1.5 mol�������������غ���n��NaOH��=2n��Na2SO4��+n��NaAlO2��=0.4L ��8 mol/L=3.2mol���ݴ˼���n��NaAlO2��=0.2mol���ٸ�����ԭ���غ�n��Al��=n��NaAlO2��=0.2mol��m��Al��=5.4g��m��Mg��=9.3g����Mgռ����Ͱٷ���Ϊ63.3%������ѡ��B��C����AD��ȷ��
���㣺���⿼����ǻ�Ͻ����ɷּ����м���ֵ����Ӧ�á�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| 900b |
| 17a |
| 900b |
| 17a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| 9b |
| 17a |
| 9b |
| 17a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 54b |
| 102a |
| 54b |
| 102a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
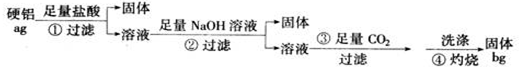
 Ϊ���о�����ijЩ��ѧ���ʼ��ⶨijþ���Ͻ�������������ͬѧ�ǽ���������ʵ�飺
Ϊ���о�����ijЩ��ѧ���ʼ��ⶨijþ���Ͻ�������������ͬѧ�ǽ���������ʵ�飺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com