
 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
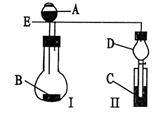
| A�����Թ�����ע������NaOH��Һ����Ȼ�������С���NaOH��Һ��ȥ����������ˮϴ���Թܱ��á� | B����ϴ�����Թ�������������Һ�� | C�����Թܱڼ�����ȩϡ��Һ�� |
| D�����ȡ���ش��������⣺ |

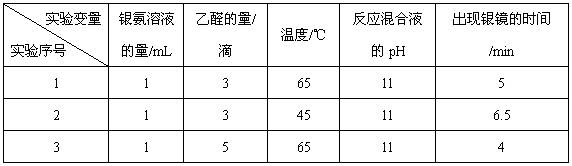

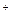 ����Һ�д���ƽ�⣺[Ag(NH3)2]
����Һ�д���ƽ�⣺[Ag(NH3)2]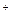
 Ag
Ag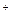 +2NH3��
+2NH3���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
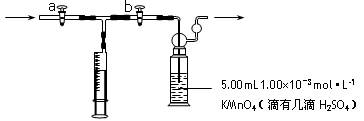
| A�������¶ȹ��߶�ʹ����ͭ���ַֽ� | B��������ˮϴ��û�к�� |
| C�����Ⱥ���ڿ�������ȴ | D����ĩδ��ȫ���ֹͣ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
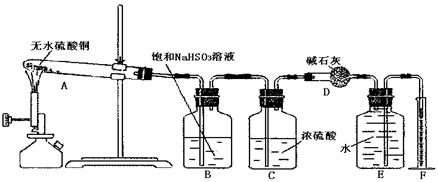
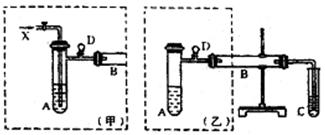
| A����ˮ����ͭδ��ȫ�ֽ�o*m |
| B��ʵ�����ʱװ��A�в��������� |
C��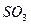 �� ��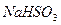 ��Һ����ʱ������ ��Һ����ʱ������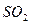 ���� ���� |
| D��������Ͳ�еĶ���ʱ��E�е�Һ�����F�е�Һ�� |
 �����������Ϊ224mL����״�������ݴ˿ɼ������������Ϊ mol����������Ϊ mol��
�����������Ϊ224mL����״�������ݴ˿ɼ������������Ϊ mol����������Ϊ mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
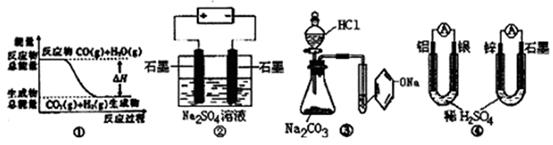
A��ͼ�ٱ�ʾ��Ӧ��CO��g��+H2O��g�� CO2��g��+H2��g���еġ�H>0 CO2��g��+H2��g���еġ�H>0 |
| B��ͼ����ͨ��ʱ������Ӧ�Ļ�ѧ����ʽΪ��Na2SO4==2Na+SO2��+O2�� |
| C��װ�âۿ����Ƚ����ᡢ̼�ᡢ��������ǿ����ʵ�� |
D��ͼ�ܵ�����װ���У����缫��п�缫����ԭ ��صĸ��� ��صĸ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ����ʵ | ���� |
| A | ��ˮ�м���ױ�����ˮ����ɫ | �ױ����巢���ӳɷ�Ӧ |
| B | �ڵ��з�̪��Na2CO3��Һ�У�����BaC12��Һ���ɫ��ȥ | ��֤Na2CO3��Һ�д���ˮ��ƽ�� |
| C | ���������Ҵ���Ӧ��������ˮ��Ӧ���� | �Ҵ������е��ǻ���ԭ�Ӳ���ˮ�����е���ԭ�ӻ��� |
| D | ʵ��ʱ��ָ�ϲ�С��մ�ϱ��� | ������70�����ϵ�ˮ��ϴ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com