
36.5 g HCl�ܽ���1 Lˮ��(ˮ���ܶȽ���Ϊ1 g��mL��1)��������Һ���ܶ�Ϊ�� g��mL��1����������ΪW�����ʵ���Ũ��Ϊc mol��L��1��NA��ʾ�����ӵ���������������������ȷ����(����)
A��������Һ�����ʵ���Ũ�ȣ�c��1 mol��L��1
B��������Һ�к���NA��HCl����
C��36.5 g HCl����ռ�е����Ϊ22.4 L
D��������Һ������������W��36.5 c/(1 000��)
D
��������A����36.5 g HCl�����ʵ�����1 mol������Һ���������1 L������������Һ�����ʵ���Ũ�Ȳ���1 mol��L��1��B����36.5 g HCl�����ʵ�����1 mol��������Һ��HCl��ȫ���룬��Һ�в�����HCl���ӣ�C������Ϊ����û�и����¶Ⱥ�ѹǿ�����Բ���ȷ��������ռ�е������Dѡ����ȷ���������ʵ���Ũ������Һ����������֮�任�����Ҫ��ʽ��Ҫ��ѧ���ܹ����ղ������á�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר��ʮһ��������Ԫ����ϰ���������棩 ���ͣ�ѡ����
��ѧ֪ʶ��������������������Ҫ��Ӧ�ã�����˵������ȷ����(����)
A������������ˮ��
B����ͭ��ˮ��ͷ���Ӵ��ĸ���ˮ��������ʴ
C�����ͷ��к��н϶��NaHCO3����ʹ���Ƴ��ĸ�����ɶ��
D�������ơ�����þ�Ȼ��ý����Ż�ʱ������ʹ����ĭ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר�������ӷ�Ӧ��ϰ���������棩 ���ͣ�ѡ����
����ȷ��ʾ���з�Ӧ�����ӷ���ʽ�ǣ� ��
A��Ũ��������м��Ӧ��2Fe��6H��=2Fe3����3H2��
B������CuSO4��Һ��Ӧ��2Na��Cu2��=Cu����2Na��
C��NaHCO3��Һ��ϡH2SO4��Ӧ��CO32-��2H��=H2O��CO2��
D����FeCl3��Һ�м���Mg(OH)2��3Mg(OH)2��2Fe3��=2Fe(OH)3��3Mg2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר��Ż�ѧ��Ӧ���ʻ�ѧƽ����ϰ���������棩 ���ͣ������
ú������Һ�����ִ���Դ��ҵ���ص㿼�ǵ���Դ�ۺ����÷������������������Ϊ��ú����ˮú��������ǰ�Ƚ����е�Һ������Ϊ��ú����CH3OH��
��1����֪��CO2(g)��3H2(g)=CH3OH(g)��H2O(g)����H1
2CO(g)��O2(g)=2CO2(g)����H2
2H2(g)��O2(g)=2H2O(g)����H3
��ӦCO(g)��2H2(g)=CH3OH(g)����H��______��
��2����ͼ�Ǹ÷�Ӧ�ڲ�ͬ�¶���CO��ת������ʱ��仯�����ߡ�
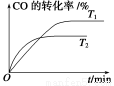
��T1��T2�¶��µ�ƽ�ⳣ����С��ϵ��K1________K2(������������������������)��
����CO�ϳɼ״�ʱ��CO��250 ����300 ����350 ���´ﵽƽ��ʱת������ѹǿ�Ĺ�ϵ��������ͼ��ʾ��������c����ʾ���¶�Ϊ________ ����ʵ����������������250 ����1.3��104 kPa���ң�ѡ���ѹǿ��������____________��
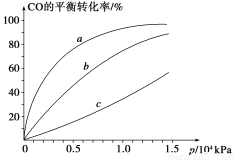
�������йظ÷�Ӧ��˵����ȷ����________(�����)��
A�����¡����������£��������ڵ�ѹǿ�������仯������淴Ӧ�ﵽƽ��
B��һ�������£�H2������������CO���������ʵ�2��ʱ�����淴Ӧ�ﵽƽ��
C��ʹ�ú��ʵĴ��������̴ﵽƽ���ʱ�䲢���CH3OH�IJ���
D��ij�¶��£���2 mol CO��6 mol H2����2 L�ܱ������У���ַ�Ӧ���ﵽƽ����c(CO)��0.2 mol��L��1����CO��ת����Ϊ80%
��3��һ���¶��£���2 L�̶�������ܱ������м���1 mol CH3OH(g)��������Ӧ��CH3OH(g)??CO(g)��2H2(g)��H2�����ʵ�����ʱ��仯��������ͼ��ʾ��

0��2 min�ڵ�ƽ����Ӧ����v(CH3OH)��__________�����¶��£���ӦCO(g)��2H2(g)??CH3OH(g)��ƽ�ⳣ��K��__________����ͬ�¶��£�����ʼʱ����CH3OH(g)�����ʵ�����ԭ����2������__________(�����)��ԭ����2����
A��ƽ�ⳣ�� B��CH3OH��ƽ��Ũ��
C���ﵽƽ���ʱ�� D��ƽ��ʱ������ܶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר��Ż�ѧ��Ӧ���ʻ�ѧƽ����ϰ���������棩 ���ͣ�ѡ����
��ӦN2O4(g)  2NO2(g)����H����57 kJ��mol��1�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯��������ͼ��ʾ������˵����ȷ���ǣ� ��
2NO2(g)����H����57 kJ��mol��1�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯��������ͼ��ʾ������˵����ȷ���ǣ� ��

A��a��c����ķ�Ӧ���ʣ�a��c
B��a��c�����������ɫ��a�cdz
C��a��b���������ƽ����Է���������a��b
D��b��c���㻯ѧƽ�ⳣ����b��c
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר������Һ��ɵļ�������Ӧ����ϰ���������棩 ���ͣ�ѡ����
3 gþ���Ͻ���100 mLϡ����ǡ����ȫ��Ӧ������Ӧ�����Һ�������ɣ��õ���ˮ������17.4 g����ԭ��������ʵ���Ũ��Ϊ(����)
A��1 mol��L��1 B��1.5 mol��L��1
C��2 mol��L��1 D��2.5 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר����ѧ��Ӧ��������ϰ���������棩 ���ͣ������
����ƽ�����ʢ��ǿ��ԭ��Һ̬��(N2H4)��ǿ������˫��ˮ�������ǻ�Ϸ�Ӧʱ������������������ˮ���������ų������ȡ���֪��0��4 molҺ̬����������˫��ˮ��Ӧ�����ɵ�����ˮ�������ų�256��652 kJ��������
��ش��������⣺
��1���÷�Ӧ���Ȼ�ѧ����ʽΪ_______________________________________________��
��2������֪H2O(l)=H2O(g)����H����44 kJ��mol��1����16 gҺ̬����˫��ˮ��Ӧ����Һ̬ˮʱ�ų���������________ kJ��
��3���˷�Ӧ���ڻ���ƽ������ͷŴ����ȺͿ��ٲ��������������һ���ܴ���ŵ���________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ����ר��ͻ�� ר��һ���ʵ�������ʺͷ�����ϰ���������棩 ���ͣ�ѡ����
�Ȼ�����Һ����������������еĹ�ͬ�����ǣ� ��
A����ɢ�ʿ���ֱ������1��100 nm֮��
B���������ɡ����պ�������������
C���ʺ��ɫ
D��������Ĥ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧר��ͻ��ѵ�� ר��7�������Һ��ϰ���������棩 ���ͣ�ѡ����
������,��100 mL 0.01 mol��L-1 HA��Һ����μ���0.02 mol��L-1 MOH��Һ,ͼ����ʾ���߱�ʾ�����Һ��pH�仯���(��Һ����仯���Բ���)������˵����,��ȷ���ǣ� ��

A.HA����ΪһԪ����
B.MOHΪһԪǿ��
C.N��ˮ�ĵ���̶�С��K��ˮ�ĵ���̶�
D.��K���Ӧ����Һ��pH=10,����c(MOH)+c(M+)=0.01 mol��L-1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com