
���� ���������֪4.66g���������ᱵ���������ټ�������еı�Ԫ�ص�������3.20g������Fe2O3����������������е���Ԫ�ص��������������������Ԫ�ص�����=12.52g-��Ԫ�ص�������2-��Ԫ�ص�������2��������Ԫ�ص���������12.52g����ѧʽʱ���ݸ�Ԫ�ص������������ԭ�����������ԭ�Ӹ����������ɣ�
��� �⣺��1���������������������Һ�õ���ɫ������������ֻ�����ᱵ���ܣ�����4.66g���������ᱵ�������������ᱵ�б�Ԫ�ص�������4.66g��$\frac{137}{233}$��100%=2.74g������������м����������Ƶõ����ɫ���������ɫ����ֻ����������������3.20g��Fe2O3����������Fe2O3����Ԫ�ص�������3.20g��$\frac{112}{160}$��100%=2.24g��������Ԫ��������12.52g-2.74g��2-2.24g��2=2.56g���ɱ�Ԫ������Ϊ��2.74g����Ԫ������Ϊ��2.24g����Ԫ������Ϊ��2.65g�����Ա���������ԭ�Ӹ�����Ϊ��$\frac{2.74}{137}$��$\frac{2.24}{56}$��$\frac{2.56}{16}$=1��2��4 ���Ի�ѧʽΪ��BaFe204��BaO•Fe203���𣺴�������������ΪBaFe204��BaO•Fe203��
��2����Ԫ����������Ϊ$\frac{2.56}{12.52}$��100%=20.45%���𣺴��Է�ĩ�����к���Ԫ�ص���������Ϊ20.45%��
���� ������Ҫ���黯ѧʽ�ļ��㷽���������Ĺؼ���Ҫ֪��4.66g���������ᱵ��������3.20g����������������������


 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
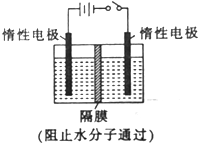 ��NO2Ϊԭ�Ͽ����Ƶ�������ɫ������N2O5��ԭ�����Ƚ�NO2ת��ΪN2O4��Ȼ����õ�ⷨ�Ʊ�N2O5����װ����ͼ��ʾ��
��NO2Ϊԭ�Ͽ����Ƶ�������ɫ������N2O5��ԭ�����Ƚ�NO2ת��ΪN2O4��Ȼ����õ�ⷨ�Ʊ�N2O5����װ����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ij��ɫϡ��ҺX�У����ܺ��б����������е�ij���֣�
ij��ɫϡ��ҺX�У����ܺ��б����������е�ij���֣�| ������ | CO32-��SiO32-��AlO2-��Cl- |
| ������ | Al3+��Fe3+��Mg2+��NH4+��Na+ |
| A�� | ��Y�����ᣬ��Oa��ת��Ϊ���������ӣ����У���ͬ��ֻ��AlO2- | |
| B�� | ��Y�����ᣬ����Һ�п��ܺ��е���������Al3+ | |
| C�� | ��Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ Al��OH��3+OH-�TAlO2-+2H2O | |
| D�� | ��Y��NaOH��Һ����X��Һ��ֻ�����������ǣ�Al3+��Fe3+��NH4+��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ˮ����ע��ʢ��һ����Ũ������ձ��У������Ͻ�������ȴ | |
| B�� | ����Ķ���������100mL��Ͳ��250mL����ƿ��������ƽ | |
| C�� | ��ȡ��������Ϊ98%��Ũ���ᣨ��=1.84g•cm-3�������Ϊ25.0mL | |
| D�� | ��������ƿ�м�������ˮ�������õ�Ũ����ע������ƿ���붨�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������ˮ���ʻ��о����������ζ | |
| B�� | ����ͼ״���һ��������Ҳ�ܷ���������Ӧ | |
| C�� | ������ӦҲ����ȡ����Ӧ | |
| D�� | ������Ӧ����Ҫϡ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 5�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3OH | B�� | CH3CH2CH3 | ||
| C�� | 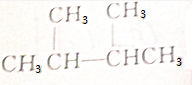 | D�� | CH3COOCH2CH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��Ԫ�ؼȱ������ֱ���ԭ | |
| B�� | �������뻹ԭ�������ʵ���֮��Ϊ1��2 | |
| C�� | ÿ����1molNa2S2O3��ת��4mol���� | |
| D�� | ��ͬ�����£�ÿ����10m3SO2�ͻ�ų�2.5m3CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
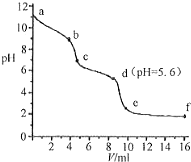 �����£�����֪Ũ�ȵ�����ζ���δ֪Ũ�ȵ�Na2CO3��Һ����pH��������û����Һ��pH�仯���ߣ���ͼ��ʾ������֪����CO2��Һ��pHΪ5.6������˵����ȷ���ǣ�������
�����£�����֪Ũ�ȵ�����ζ���δ֪Ũ�ȵ�Na2CO3��Һ����pH��������û����Һ��pH�仯���ߣ���ͼ��ʾ������֪����CO2��Һ��pHΪ5.6������˵����ȷ���ǣ�������| A�� | a��ʱ����Һ�ʼ��Ե�ԭ����CO32-����ˮ�ⷴӦ�������ӷ���ʽΪ��CO32-+2H2O=H2CO3+2OH- | |
| B�� | a��b�Σ���Һ������ų� | |
| C�� | c���Ժ�������� | |
| D�� | d����Һ��c��Na+��=c��Cl-�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com