
A��B��C��D���ֿ��ܵĻ�����������Ӹ�����ͬ�����ֱ���������Na����Mg2����Al3����Ba2����������OH����Cl����SO42-��CO32-������϶��ɡ�Ϊ��ȷ�������ֻ�����ijɷ֣�ijͬѧ����������ʵ�������
�ٽ����ֻ������ȡ���������Һ���ֱ�װ����֧�Թܡ�
��ȡA��Һ�ֱ��������������Һ�У���¼ʵ���������£�
B��Һ ��ɫ����
��ɫ����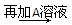 �������ܽ�
�������ܽ�
C��Һ ��ɫ����
��ɫ����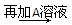 �������ܽ�
�������ܽ�
D��Һ ��ɫ����
��ɫ����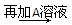 ���������ܽ�
���������ܽ�
����B��Һ�е���D��Һ��������ʵ������
��ش��������⣺
��1��д�����ǵĻ�ѧʽ��A________��B________��C________��D________��
��2�������ڵĵ�����ʵ�飬�ټ���A�����������ܽ�����ӷ���ʽΪ_________________________________________________________________��
��3����������C��Һ�е���D��Һ�����ܳ��ֵ�ʵ��������_________________________________________________________________��
�����ӷ���ʽ��ʾ��ԭ��_________________________________________��
 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ɫ��Һ����Na����Ag����Ba2����Al3����AlO2����MnO4����CO32����SO42����SiO32���е���
�������(������ˮ�ĵ���)��ȡ����Һ��������ʵ�飺
��.ȡ������Һ��������������ᣬ���������ɣ����õ���ɫ��Һ��
��.�ڢ�������Һ�м��������NH4HCO3��Һ�����������ɣ�ͬʱ������ɫ�����ף�
��.�ڢ�������Һ�м��������Ba(OH)2��Һ�����ȣ�Ҳ���������ɣ�ͬʱ������ɫ�����ҡ�
��ش��������⣺
(1)��ʵ����֪ԭ��Һ��һ�������е�������________��һ�����е�������________________��
(2)��ʵ����֪ԭ��Һ�л�һ�����е�������________�����ɼ����ӷ���ʽΪ________________��
(3)ʵ��������ɰ�ɫ�����ҵ����ӷ���ʽΪ________________��
(4)ԭ��Һ�л����ܴ��ڵ�������________����������ӵķ�����_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ú���Al2O3��SiO2������FeO��xFe2O3�������Ʊ�Al2(SO4)3��18H2O��������������(���ֲ�����������)��
��.�������м������ϡH2SO4�����ˣ�
��.����Һ�м������KMnO4��Һ��������Һ��pHԼΪ3��
��.���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ��
��������MnSO4���Ϻ�ɫ��ʧ�����ˣ�
V��Ũ�����ᾧ�����룬�õ���Ʒ��
(1)H2SO4�ܽ�Al2O3�����ӷ���ʽ��_______________________��
(2)��MnO4������Fe2�������ӷ���ʽ����������
1MnO4����Fe2����________===1Mn2����Fe3����________
(3)��֪��
�����������������pH
| | Al(OH)3 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������Ԫ����һ���������ԣ�FeCl3��Һ����һ����SO2����Һ��ɫ�����仯��
(1)��Ӧ�����ӷ���ʽΪ_________________________________________________��
(2)��H����OH����H2O�⣬��Һ��һ�����е�����________��
| A��Fe2�� | B��Fe3�� | C��Cl�� | D��SO42����E��H2SO3 |
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1�� | |
| ����2�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ�ְ�ɫ��ĩ���������������Ӻ��������еļ��֡�
�����ӣ�S2����Cl����NO3����SO42����CO32����HCO3����MnO4����
�����ӣ�Na����Mg2����Al3����Ba2����Fe2����Fe3����Cu2����NH4+��
���ð�ɫ��ĩ��������ʵ�飬�۲쵽���������£�
| ʵ����� | ���� |
| a.ȡ������ĩ����ˮ���� | ȫ���ܽ⡢ |
| ��Һ��ɫ�� | |
| b.��������Һ�����������������Һ�������� | ���������� |
| c.ȡ������ĩ�������� | ���������� |
| d.ȡ������ĩ����ϡH2SO4��ϡHNO3�Ļ��Һ | �а�ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ʵ���Һ�ũҵ�������й㷺����;��ʵ�����Զ�������Ϊ��Ҫԭ���Ʊ�������ء��䲿���������£�
��1���ڢٲ���������Ӧ�� �н��У������ô�������ԭ���� ���û�ѧ��ʽ��ʾ����
��2���ڢܲ�ͨ��CO2������ʹMnƬ������Ӧ������MnO4����MnO2����Ӧ�����ӷ���ʽΪ ������ɷ�Ӧʱ��ת��ΪKMnO4��ռȫ��K2MnO4�İٷ���ԼΪ ����ȷ��0��1%����
��3���ڢݲ����ȹ��˵��� ��
��4���ڢ�����Ũ����Һ����ϸС��������ʱ��ֹͣ���ȣ���ȴ�ᾧ�� ��ϴ�ӡ������������У��¶Ȳ��˹��ߣ���ԭ���� ���û�ѧ����ʽ��ʾ����
��5��H2O2��KMnO4�����dz��õ�ǿ������������H2O2��Һ�еμ����Ը��������Һ�������Ը��������Һ����ɫ��д���÷�Ӧ�����ӷ���ʽ�� ���÷�Ӧ˵��H2O2�������Ա�KMnO4 ���ǿ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
1 L ij�����Һ�����ܺ��е��������±���
| ���ܴ������е������� | H+��K+��Mg2+��Al3+��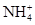 ��Fe2+��Fe3+ ��Fe2+��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��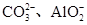 |

| Cl2�����(��״��) | 2.8 L | 5.6 L | 11.2L |
| n(Cl-) | 1.25 mol | 1.5 mol | 2 mol |
| n(Br-) | 1.5 mol | 1.4 mol | 0.9 mol |
| n(I-) | a mol | 0 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������(HNO2)��һ������������൱������,�ܲ��ȶ�,ͨ���������������ֽ⡣
(1)д��������ĵ��뷽��ʽ:��������������������
(2)������������,��NaNO2��KI�����ʵ���֮��1��1ǡ����ȫ��Ӧ,��I-������ΪI2ʱ,�����к���������Ϊ��������(�ѧʽ)��
(3)Ҫ�õ��ȶ���HNO2��Һ,�������䶳��NaNO2Ũ��Һ�м����ͨ��ij������,�������ʲ��ʺϵ�����������(����ĸ)��
a.ϡ���ᡡb.������̼��c.��������d.����
(4)���Թ�ҵ��ˮ�е�N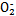 �������۳�ȥ����֪�˷�Ӧ��ϵ�к���Al��NaAlO2��NaNO2��NaOH��NH3��H2O��H2O�������ʡ�
�������۳�ȥ����֪�˷�Ӧ��ϵ�к���Al��NaAlO2��NaNO2��NaOH��NH3��H2O��H2O�������ʡ�
��д��������Ӧ�����ӷ���ʽ:��������������������������������
�ڷ���������Ӧ���ˮ��pH����������(�������С�����䡱)��
(5)���Թ�ҵ��ˮ�е�N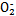 �������س�ȥ������
�������س�ȥ������
(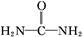 )��N
)��N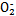 �����������·�Ӧ�������������塣�÷�Ӧ������16.8 L(������Ϊ��״��)�������ʱ,�������ص�����Ϊ�������� g��
�����������·�Ӧ�������������塣�÷�Ӧ������16.8 L(������Ϊ��״��)�������ʱ,�������ص�����Ϊ�������� g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��X��Y��Z��W�������ʵ�ˮ��Һ�����Ƿֱ���Na2CO3��NaOH��CH3COOH��NaCl�е�һ�֡���֪X��Y����Һ��ˮ�ĵ���̶���ͬ��X��Z����Һ��pH��ͬ����ش��������⣺
��1��X�� ��Z�� ��
��2��Z��W����Һ��ˮ�ĵ���̶��ɴ�С��˳��Ϊ ���û�ѧʽ��ʾ����
��3��д��Z��Һ�����Y��Һ��Ӧ�����ӷ���ʽ ��
��4��X��Y����Һǡ����ȫ��Ӧ����Һ�и�����Ũ���ɴ�С��˳����
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com