
����ͭ���壨CuSO4?xH2O���ᾧˮ�����IJⶨʵ�顣
�ٳ���һ����������Ǧ��������ѽᾧˮʱ�õ���������Ҫ�У������������Ǽܡ�����������ǯ�������Ǻ� ��
��ʵ����Ҫ���������ڸ���������ȴ������ҪĿ����
��
��������������Ϊm������������ͭ���������Ϊm1�����Ⱥ���������ˮ����ͭ������Ϊm2������CuSO4?xH2O��x= ��д����ʽ����
�����ʵ���м����¶ȹ��ߣ�������ɫ��ڣ���ⶨ����� ������䡱��ƫ�ߡ���ƫ�͡���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ��ʼ������pH | ��ȫ������pH | Fe2+ | 6.4 | 8.4 | Fe3+ | 2.4 | 3.1 | Cu2+ | 5.2 | 6.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
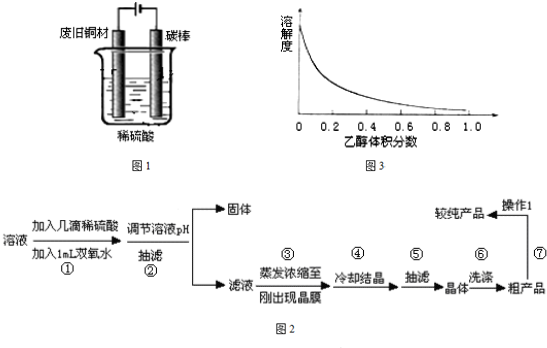
 ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
| ||
| ||
| �������� | �����뾧��������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.9g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����ǰ������ | ���Ⱥ������ | |
| m0�������� | m1������+���壩 | m2������+��ˮ����ͭ�� |
| 18.269g | 19.420g | 19.011g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��
ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��| �������� | �����뾧�������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.6g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com