
баОПаЁзщгћНјааДгКЃЫЎжаЛёШЁЕЫЎЁЂЪГбЮВЂЬсШЁУОКЭфхЕШЮяжЪЕФЪЕбщЬНОПЁЃ
ЃЈ1ЃЉЮоашОЙ§ЛЏбЇБфЛЏОЭФмДгКЃЫЎжаЛёЕУЕФвЛзщЮяжЪЪЧЁЁЁЁЁЁЁЁЁЁ ЃЈЬюађКХЃЉЁЃ
Ђй Cl2ЁЂBr2ЁЂI2ЁЁЁЁ Ђк NaЁЂMgЁЂAlЁЁ ЂлЩеМюЁЂЧтЦјЁЁЁЁЁЁ ЂмЪГбЮЁЂЕЫЎ
ЃЈ2ЃЉдкЩњВњЙ§ГЬжаЃЌДгКЃЫЎжаЬсШЁУОЕФСїГЬШчЯТЭМЫљЪОЃК
 |
ЗДгІЂкЕФЛЏбЇЗНГЬЪНЮЊЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁ ЁЃ
ЃЈ3ЃЉЪЕбщЪвДгКЃЫЎбљЦЗжаЬсШЁфхЕФжївЊВНжшЪЧЃКЯђКЃЫЎбљЦЗжаЭЈШыЪЪСПТШЦјНЋфхРызгбѕЛЏЃЌИУЗДгІЕФРызгЗНГЬЪНЮЊЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁ ЃЛДгЗДгІКѓЕФШмвКжаЬсШЁфхЕЅжЪЕФЪЕбщВйзїЪЧ____________ЃЈЬюЪЕбщВйзїУћГЦЃЉЁЃ

| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2015НьЫФДЈЪЁзЪбєЪаИпвЛЯТбЇЦкЦкФЉМьВтЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
баОПаЁзщгћНјааДгКЃЫЎжаЛёШЁЕЫЎЁЂЪГбЮВЂЬсШЁУОКЭфхЕШЮяжЪЕФЪЕбщЬНОПЁЃ
ЃЈ1ЃЉЮоашОЙ§ЛЏбЇБфЛЏОЭФмДгКЃЫЎжаЛёЕУЕФвЛзщЮяжЪЪЧ ЃЈЬюађКХЃЉЁЃ
Ђй Cl2ЁЂBr2ЁЂI2 Ђк NaЁЂMgЁЂAl ЂлЩеМюЁЂЧтЦј ЂмЪГбЮЁЂЕЫЎ
ЃЈ2ЃЉдкЩњВњЙ§ГЬжаЃЌДгКЃЫЎжаЬсШЁУОЕФСїГЬШчЯТЭМЫљЪОЃК
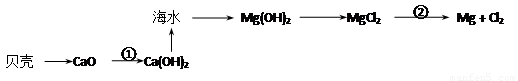
БДПЧжаЕФжївЊГЩЗжЪЧЃК___________ЃЈЬюЛЏбЇЪНЃЉЃЛЗДгІЂйЪєгк________ЗДгІЃЈЬюЁАЮќШШЁБЛђЁАЗХШШЁБЃЉЁЃ
ЗДгІЂкЕФЛЏбЇЗНГЬЪНЮЊ ЁЃ
ЃЈ3ЃЉЪЕбщЪвДгКЃЫЎбљЦЗжаЬсШЁфхЕФжївЊВНжшЪЧЃКЯђКЃЫЎбљЦЗжаЭЈШыЪЪСПТШЦјНЋфхРызгбѕЛЏЃЌИУЗДгІЕФРызгЗНГЬЪНЮЊ ЃЛДгЗДгІКѓЕФШмвКжаЬсШЁфхЕЅжЪЕФЪЕбщВйзїЪЧ____________ЃЈЬюЪЕбщВйзїУћГЦЃЉЁЃ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com