
ÓŠM”¢NĮ½ÖÖČÜŅŗ£¬¾²ā¶ØÕāĮ½ÖÖČÜŅŗÖŠŗ¬ÓŠĻĀĮŠ12ÖÖĄė×Ó£ŗAl3£«”¢Cl£”¢Na£«”¢K£«”¢NO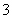 ”¢OH£”¢Fe2£«”¢AlO
”¢OH£”¢Fe2£«”¢AlO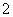 ”¢CO
ӢCO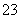 ӢNH
ӢNH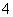 ӢSO
”¢SO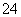 ”¢H£«”£
”¢H£«”£
(1)Ķź³ÉĻĀĮŠ±ķøńÖŠŹµŃé¢ŁµÄ½įĀŪŗĶŹµŃé¢ŚµÄŹµŃéÄŚČŻŅŌ¼°ĻÖĻó£ŗ
| ŹµŃéÄŚČŻŅŌ¼°ĻÖĻó | ½įĀŪ |
| ¢ŁČ”ÉŁĮæNČÜŅŗµĪ¼Ó×ćĮæµÄĻõĖį±µČÜŅŗ£¬ĪŽ³Įµķ²śÉś | |
| ¢Ś | Č·¶ØMČÜŅŗÖŠŗ¬ÓŠNa£«£¬²»ŗ¬K£« |
| ¢ŪÓĆpHŹŌÖ½¼ģ²āMČÜŅŗ£¬pHŹŌÖ½³ŹĄ¶É« |
(2)øł¾Ż(1)ÖŠµÄŹµŃé»Ų“š£ŗ
NO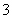 “ęŌŚÓŚ________ČÜŅŗÖŠ£¬ĄķÓÉŹĒ________________________________________________________________________£»
“ęŌŚÓŚ________ČÜŅŗÖŠ£¬ĄķÓÉŹĒ________________________________________________________________________£»
Cl£“ęŌŚÓŚ________ČÜŅŗÖŠ£¬ĄķÓÉŹĒ________________________________________________________________________”£
(3)øł¾Ż(1)ÖŠµÄŹµŃéČ·¶Ø£¬MČÜŅŗÖŠŗ¬ÓŠµÄĄė×ÓĪŖ________________________________________________________________________”£
“š°ø””(1)¢ŁNČÜŅŗÖŠ²»ŗ¬CO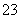 ”¢SO
”¢SO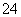 (»ņMČÜŅŗÖŠŅ»¶Øŗ¬ÓŠCO
(»ņMČÜŅŗÖŠŅ»¶Øŗ¬ÓŠCO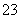 ”¢SO
ӢSO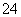 )
)
¢ŚČ”MČÜŅŗ½ųŠŠŃęÉ«·“Ó¦£¬ŃęÉ«ĪŖ»ĘÉ«£¬ŌŁĶø¹żĄ¶É«īܲ£Į§¹Ū²ģ»šŃęŃÕÉ«£¬²»³Ź×ĻÉ«
(2)M””NČÜŅŗÖŠŗ¬ÓŠH£«”¢Fe2£«”¢Al3£«”¢NH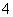 ”¢K£«£¬ÓÉÓŚNČÜŅŗĪŖĖįŠŌ£¬ÓÖŗ¬ÓŠFe2£«£¬ĖłŅŌNČÜŅŗÖŠ²»ŗ¬NO
”¢K£«£¬ÓÉÓŚNČÜŅŗĪŖĖįŠŌ£¬ÓÖŗ¬ÓŠFe2£«£¬ĖłŅŌNČÜŅŗÖŠ²»ŗ¬NO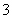
N””øł¾ŻČÜŅŗ³ŹµēÖŠŠŌŌŌņ£¬æÉŅŌČ·¶ØCl£“ęŌŚÓŚNČÜŅŗÖŠ
(3)OH£”¢AlO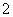 ”¢CO
ӢCO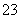 ӢSO
”¢SO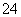 ”¢Na£«”¢NO
”¢Na£«”¢NO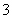
½āĪö””ÉŁĮæNČÜŅŗÖŠµĪ¼Ó×ćĮæµÄĻõĖį±µČÜŅŗ£¬ĪŽ³Įµķ²śÉś£¬ĖµĆ÷NČÜŅŗÖŠ²»ŗ¬CO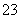 ”¢SO
”¢SO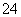 £¬ÄĒĆ“MČÜŅŗÖŠŅ»¶Øŗ¬ÓŠCO
£¬ÄĒĆ“MČÜŅŗÖŠŅ»¶Øŗ¬ÓŠCO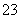 ”¢SO
”¢SO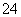 ”£Č”MČÜŅŗ½ųŠŠŃęÉ«·“Ó¦£¬ŃęÉ«ĪŖ»ĘÉ«£¬Ö¤Ć÷ŗ¬ÓŠNa£«£¬ŌŁĶø¹żĄ¶É«īܲ£Į§¹Ū²ģ»šŃęŃÕÉ«£¬²»³Ź×ĻÉ«£¬ĖµĆ÷MÖŠ²»ŗ¬K£«£»ÓĆpHŹŌÖ½¼ģ²āMČÜŅŗ£¬pHŹŌÖ½³ŹĄ¶É«£¬ĖµĆ÷MČÜŅŗĻŌ¼īŠŌ£¬ŗ¬ÓŠ“óĮæµÄOH££¬ÄĒĆ“NČÜŅŗÖŠŗ¬ÓŠ“óĮæµÄH£«”£AlO
”£Č”MČÜŅŗ½ųŠŠŃęÉ«·“Ó¦£¬ŃęÉ«ĪŖ»ĘÉ«£¬Ö¤Ć÷ŗ¬ÓŠNa£«£¬ŌŁĶø¹żĄ¶É«īܲ£Į§¹Ū²ģ»šŃęŃÕÉ«£¬²»³Ź×ĻÉ«£¬ĖµĆ÷MÖŠ²»ŗ¬K£«£»ÓĆpHŹŌÖ½¼ģ²āMČÜŅŗ£¬pHŹŌÖ½³ŹĄ¶É«£¬ĖµĆ÷MČÜŅŗĻŌ¼īŠŌ£¬ŗ¬ÓŠ“óĮæµÄOH££¬ÄĒĆ“NČÜŅŗÖŠŗ¬ÓŠ“óĮæµÄH£«”£AlO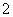 ²»æÉÄÜ“ęŌŚÓŚĖįŠŌČÜŅŗÖŠ£¬Al3£«”¢Fe2£«”¢NH
²»æÉÄÜ“ęŌŚÓŚĖįŠŌČÜŅŗÖŠ£¬Al3£«”¢Fe2£«”¢NH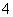 ²»æÉÄÜ“ęŌŚÓŚ¼īŠŌČÜŅŗÖŠ£¬ĖłŅŌÅŠ¶ĻMČÜŅŗÖŠÓŠOH£”¢AlO
²»æÉÄÜ“ęŌŚÓŚ¼īŠŌČÜŅŗÖŠ£¬ĖłŅŌÅŠ¶ĻMČÜŅŗÖŠÓŠOH£”¢AlO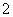 ”¢CO
ӢCO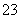 ӢSO
”¢SO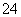 ”¢Na£«£¬NČÜŅŗÖŠŗ¬ÓŠH£«”¢Fe2£«”¢Al3£«”¢NH
”¢Na£«£¬NČÜŅŗÖŠŗ¬ÓŠH£«”¢Fe2£«”¢Al3£«”¢NH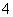 ”¢K£«”£ÓÉÓŚNČÜŅŗĪŖĖįŠŌ£¬ÓÖŗ¬ÓŠFe2£«£¬ĖłŅŌNČÜŅŗÖŠ²»ŗ¬NO
”¢K£«”£ÓÉÓŚNČÜŅŗĪŖĖįŠŌ£¬ÓÖŗ¬ÓŠFe2£«£¬ĖłŅŌNČÜŅŗÖŠ²»ŗ¬NO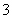 £»øł¾ŻČÜŅŗ³ŹµēÖŠŠŌŌŌņ£¬æÉŅŌČ·¶ØCl£“ęŌŚÓŚNČÜŅŗÖŠ”£
£»øł¾ŻČÜŅŗ³ŹµēÖŠŠŌŌŌņ£¬æÉŅŌČ·¶ØCl£“ęŌŚÓŚNČÜŅŗÖŠ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ¹ŲÓŚĮ£×Ó½į¹¹µÄĆčŹö²»ÕżČ·µÄŹĒ(””””)
A£®H2SŗĶNH3¾łŹĒ¼Ūµē×Ó×ÜŹżĪŖ8µÄ¼«ŠŌ·Ö×Ó
B£®HS£ŗĶHCl¾łŹĒŗ¬ÓŠŅ»øö¼«ŠŌ¼üµÄ18µē×ÓĮ£×Ó
C£®CH2Cl2ŗĶCCl4¾łŹĒĖÄĆęĢå¹¹ŠĶµÄ·Ē¼«ŠŌ·Ö×Ó
D£®1 mol D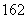 OÖŠŗ¬ÖŠ×Ó”¢ÖŹ×Ó”¢µē×Óø÷10NA(NA“ś±ķ°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµ)
OÖŠŗ¬ÖŠ×Ó”¢ÖŹ×Ó”¢µē×Óø÷10NA(NA“ś±ķ°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµ)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
X”¢Y”¢Z”¢WŹĒŌŖĖŲÖÜĘŚ±ķĒ°ĖÄÖÜĘŚÖŠµÄĖÄÖÖ³£¼ūŌŖĖŲ£¬ĘäĻą¹ŲŠÅĻ¢ČēĻĀ±ķ£ŗ
| ŌŖĖŲ | Ļą¹ŲŠÅĻ¢¶ØŠŌ“󊔹ŲĻµĪŖ ______________(ĢīŠ“»ÆѧŹ½)”£ |
| X | XµÄ»łĢ¬Ō×ÓŗĖĶā3øöÄܼ¶ÉĻÓŠµē×Ó£¬ĒŅĆæøöÄܼ¶ÉĻµÄµē×ÓŹżĻąµČ |
| Y | ³£ĪĀ³£Ń¹ĻĀ£¬Yµ„ÖŹŹĒµ»ĘÉ«¹ĢĢ壬³£ŌŚ»šÉ½æŚø½½ü³Į»ż |
| Z | ZŗĶYĶ¬ÖÜĘŚ£¬ZµÄµēøŗŠŌ“óÓŚY |
| W | WµÄŅ»ÖÖŗĖĖŲµÄÖŹĮæŹżĪŖ63£¬ÖŠ×ÓŹżĪŖ34 |
(1)YĪ»ÓŚŌŖĖŲÖÜĘŚ±ķµŚ______ÖÜĘŚµŚ______×壬YŗĶZµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļµÄĖįŠŌ½ĻĒæµÄŹĒ____________(Š“»ÆѧŹ½)”£
(2)XY2ŹĒŅ»ÖÖ³£ÓƵÄČܼĮ£¬XY2µÄ·Ö×ÓÖŠ“ęŌŚ______øö¦Ņ¼ü”£ŌŚH”ŖY£¬H”ŖZĮ½ÖÖ¹²¼Ū¼üÖŠ£¬¼üµÄ¼«ŠŌ½ĻĒæµÄŹĒ________£¬¼ü³¤½Ļ³¤µÄŹĒ_________”£
(3)WµÄ»łĢ¬Ō×ÓŗĖĶāµē×ÓÅŲ¼Ź½ŹĒ______________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻņijČÜŅŗÖŠµĪ¼ÓĀČĖ®£¬ŌŁ¼ÓČėKSCNČÜŅŗ£¬ČÜŅŗ±äŃŖŗģÉ«£¬ŌņČÜŅŗÖŠŅ»¶Ø“ęŌŚFe2£«£¬øĆÅŠ¶ĻŹĒ·ńÕżČ·£æĪŖŹ²Ć“£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
³£ĪĀĻĀ£¬ĻĀĮŠø÷×éĄė×ÓŌŚÖø¶ØČÜŅŗÖŠŅ»¶ØÄÜ“óĮæ¹²“ęµÄŹĒ(””””)
A£®1.0 mol·L£1µÄKNO3ČÜŅŗ£ŗH£«”¢Fe2£«”¢Cl£”¢SO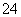
B£®¼×»ł³Č³ŹŗģÉ«µÄČÜŅŗ£ŗNH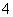 ”¢Ba2£«”¢AlO
”¢Ba2£«”¢AlO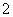 ”¢Cl£
”¢Cl£
C£®pH£½12µÄČÜŅŗ£ŗK£«”¢Na£«”¢CH3COO£ ”¢Br£
D£®ÓėĀĮ·“Ó¦²śÉś“óĮæĒāĘųµÄČÜŅŗ£ŗNa£«”¢K£«”¢CO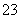 ”¢NO
ӢNO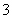
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ė®ČÜŅŗÖŠÄÜ“óĮæ¹²“ęµÄŅ»×éĄė×ÓŹĒ(””””)
A£®Al3£«”¢Cl£”¢AlO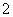 ”¢SiO
ӢSiO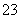
B£®H£«”¢Na£«”¢S2£”¢ClO£
C£®K£«”¢Mg2£«”¢SO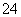 ”¢MnO£4
”¢MnO£4
D£®Fe3£«”¢Ca2£«”¢SCN£”¢NO
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«µČĪļÖŹµÄĮæµÄĆ¾ŗĶĀĮ»ģŗĻ£¬Č”µČÖŹĮæøĆ»ģŗĻĪļĖÄ·Ż£¬·Ö±š¼Óµ½×ćĮæµÄĻĀĮŠČÜŅŗÖŠ£¬³ä·Ö·“Ó¦ŗ󣬷ųöĒāĘų×ī¶ąµÄŹĒ(””””)
A£®3 mol·L£1 HCl B£®4 mol·L£1 HNO3
C£®8 mol·L£1 NaOH D£®18 mol·L£1 H2SO4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
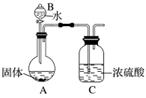
Ć¾ŌŚæÕĘųÖŠČ¼ÉÕ³żÉś³ÉMgOĶā£¬»¹æÉÄÜÉś³ÉMg3N2”£Ä³Š£»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§ĄūÓĆĆ¾ŌŚæÕĘųÖŠČ¼ÉÕŗóµÄ¹ĢĢå(²»ŗ¬µ„ÖŹ)½ųŠŠŹµŃ飬Ģ½¾æĘä×é³É”£
(1)¼××éĶ¬Ń§Č”Ņ»¶ØĮæČ¼ÉÕŗóµÄ¹ĢĢåĶ¶ČėĖ®ÖŠ£¬µĆµ½ĮĖŅ»ÖÖÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬øĆĘųĢåµÄ»ÆѧŹ½ĪŖ____________________£¬ĖµĆ÷¹ĢĢåÖŠŗ¬ÓŠMg3N2£¬Éś³ÉøĆĘųĢåµÄ»Æѧ·½³ĢŹ½ĪŖ________________________________________________________________________
________________________________________________________________________ӣ
(2)ŅŅ×éĶ¬Ń§ĪŖ²ā¶ØMg3N2µÄŗ¬Į棬ÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ½ųŠŠŹµŃ飬³ä·Ö·“Ó¦ŗóŌŁ¼ÓČČA”£ĘäÖŠÅØĮņĖįµÄ×÷ÓĆŹĒ__________________________________________________________________
______________________________________£¬¶ŌA¼ÓČȵÄÄæµÄŹĒ________________________
________________________________________________________________________ӣ
ŅŃÖŖ¼ÓČėµÄ¹ĢĢåÖŹĮæĪŖ4.0 g£¬×īÖÕC×°ÖĆŌöÖŲa g£¬Ōņ¹ĢĢåÖŠŗ¬Mg3N2______ g(ÓĆŗ¬aµÄŹ½×Ó±ķŹ¾)”£
(3)±ū×éÓŠµÄĶ¬Ń§ČĻĪŖŅŅ×éĶ¬Ń§µÄ²ā¶Ø½į¹ūĘ«øߣ¬ĄķÓÉŹĒ_______________________”£
ÓŠµÄĶ¬Ń§ČĻĪŖŅŅ×éĶ¬Ń§µÄ²ā¶Ø½į¹ūĘ«µĶ£¬ĄķÓÉŹĒ________________________________________________________________________
________________________________________________________________________ӣ
±ū×éĶ¬Ń§½ųŠŠĮĖøĽų£¬ĖūĆĒ½«ŅŅ×éĶ¬Ń§ŹµŃéÖŠµĆµ½µÄÄŃČܹĢĢå½ųŠŠ¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ£¬²¢×ĘÉÕ¹ĢĢåÖĮŗćÖŲ£¬²āµĆĘäÖŹĮæĪŖ4.08 g”£ÉĻŹö¹ż³ĢÖŠ£¬Ļ“µÓ³ĮµķµÄ²Ł×÷ŹĒ________________________________________________________________________
________________________________________________________________________ӣ
Ć¾ŌŚæÕĘųÖŠČ¼ÉÕŗóÉś³ÉµÄ¹ĢĢåÖŠMg3N2µÄÖŹĮæ·ÖŹżĪŖ______________”£
(4)ÓŠŅ»ÖÖÓĆļ§ŃĪÓėŗ¬Ć¾æóŹÆ»ģŗĻģŃÉÕÖĘČ”Ńõ»ÆĆ¾µÄ·½·Ø£¬½ā¾öĮĖĻÖÓŠ·½·Ø“ęŌŚµÄŌĮĻ³É±¾øß”¢ĻīÄæĶ¶×Ź“ó”¢ÄÜŗÄøß”¢ø±²śĘ·²»ŗĆÓƵČĪŹĢā£¬ĘäŌĄķŹĒ½«ŗ¬Ć¾æóŹÆ·Ū(ŗ¬Ńõ»ÆĆ¾)Óėļ§ŃĪ»ģŗĻ£¬¾¹żģŃÉÕ”¢Ė®ČÜ”¢¹żĀĖ£¬µĆµ½“ÖĆ¾ŃĪČÜŅŗ£¬²¢»ŲŹÕģŃÉÕ²śÉśµÄ°±”£Š“³öÓĆ¹ĢĢå(NH4)2SO4Óėŗ¬Ć¾æóŹÆ·Ū»ģŗĻģŃÉÕŹ±µÄ»Æѧ·“Ó¦·½³ĢŹ½____________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com