
 ��1����֪��
��1����֪�� H1=" 1175.7" kJ��mol-1
H1=" 1175.7" kJ��mol-1 PtF6(g) + e- = PtF6-(g)
PtF6(g) + e- = PtF6-(g)
 H2=" -" 771.1 kJ��mol-1
H2=" -" 771.1 kJ��mol-1 O2+ PtF6-(s) = O2+(g) + PtF6-
O2+ PtF6-(s) = O2+(g) + PtF6-  H3="482.2" kJ��mol-1
H3="482.2" kJ��mol-1  ��ӦO
��ӦO 2��g��+ PtF6 (g) = O2+PtF6- (s)
2��g��+ PtF6 (g) = O2+PtF6- (s)  H="_____________" kJ��mol-1
H="_____________" kJ��mol-1 J���÷�Ӧ���Ȼ�ѧ����ʽΪ________��
J���÷�Ӧ���Ȼ�ѧ����ʽΪ________��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��-386 kJ��mol-1 | B��+386 kJ��mol-1 |
| C��-746 kJ��mol-1 | D��+746 kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
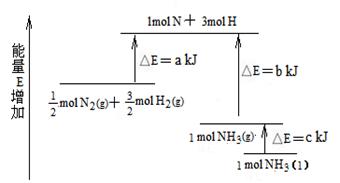
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 5OH(1)��ȫȼ������H2O(1)����ų�������Ϊ
5OH(1)��ȫȼ������H2O(1)����ų�������Ϊ �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���缫����ʽ��Cu-2e- = Cu2+�����ܷ�����ԭ����У����ܷ����ڵ����� |
| B��ij�¶��£�10mL 0.1mol/L��H2SO4��Һ��10mL 0.4 mol/L��KOH��Һ��Ϻ�pH=13 |
| C������1mol H-H��N-H��N��N�������յ������ֱ�Ϊa kJ��b kJ��c kJ�� ��2NH3( g) 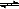 3H2(g)��N2(g) ��H="(3a+c-6b)" kJ/mol 3H2(g)��N2(g) ��H="(3a+c-6b)" kJ/mol |
| D���к͵�����Ĵ�����Һ�����ĵ�pHֵ�İ�ˮ������������Һ������ֱ�ΪV1��V2����V1<V2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 =CO2(g) ��H2=-393.5kJ/mol
=CO2(g) ��H2=-393.5kJ/mol �Լ��㷴Ӧ2C(s)+2H2(g)+O2(g) ==CH3COOH(l)���ʱ䦤H=
�Լ��㷴Ӧ2C(s)+2H2(g)+O2(g) ==CH3COOH(l)���ʱ䦤H= �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��S(s)��O2(g) �� SO2(g)�� Q1 kJ�� S(g)��O2(g) �� SO2(g)�� Q1 kJ�� S(g)��O2(g) �� SO2(g)�� Q2kJ �� SO2(g)�� Q2kJ |
B��2H2(g)��O2(g) �� 2H2O(l)��Q1 kJ ��2H2(g)��O2(g) �� 2H2O(g)��Q2 kJ �� 2H2O(l)��Q1 kJ ��2H2(g)��O2(g) �� 2H2O(g)��Q2 kJ |
C��NaOH(aq)��HCl(aq) �� NaCl(aq)��H2O(l)��Q1 kJ �� NaCl(aq)��H2O(l)��Q1 kJNaOH(aq)��CH3COOH(aq) ��CH3COONa(aq)��H2O(l)��Q2 kJ |
D��H2(g)��Cl2(g) �� 2HCl(g)��Q1 kJ�� H2(g)��F2(g) �� 2HCl(g)��Q1 kJ�� H2(g)��F2(g) �� 2HF(g)��Q2 kJ �� 2HF(g)��Q2 kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH3CH2OH��l��+3O2��g��=2CO2��g��+ 3H2O��l������H=+1367kJ/mol |
| B��2CH3CH2OH��l��+6O2��g��=4CO2��g��+ 6H2O��l������H=��2734kJ/mol |
| C��2CH3CH2OH��l��+6O2��g��=4CO2��g��+ 6H2O��l������H=��1367kJ/mol |
| D��2CH3CH2OH��l��+6O2��g��=4CO2��g��+ 6H2O��l������H=+2734kJ/mol |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com