
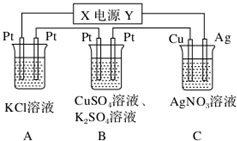
���� ��1����ͭ�缫���������ӣ���Cu�缫Ϊ��������֪XΪ��Դ�ĸ�����
��2��B�е������ͭ��Һ�������ᣬpH��С������C�е缫��Ӧ�жϣ�
��3�����ݵ缫��Ӧ�������غ������㣻
��4������A�еĵ缫��Ӧ����C��ת�Ƶĵ����غ������㣮
��� �⣺��1����ͭ�缫���������ӣ�����Ag++e-�TAg����Cu�缫Ϊ������AgΪ������YΪ��������֪XΪ��Դ�ĸ������ʴ�Ϊ��������
��2��A���Ȼ��ص��õ��������غ���������������Һ������ǿ��B�е������ͭ��Һ�������ᣬ��Һ��������Ũ������pH��С��C��������ӦΪAg++e-�TAg��������ӦΪAg-e-�TAg+����ҺŨ�Ȳ��䣬��pH���䣬
�ʴ�Ϊ������С�����䣻
��3��C��������ӦΪAg++e-�TAg��n��Ag��=$\frac{2.16g}{108g/mol}$=0.02mol����ת�Ƶĵ���Ϊ0.02mol��
B��������ӦΪ4OH--4e-�T2H2O+O2������ת��0.02mol������������Ϊ0.005mol�������Ϊ0.005mol��22.4L/mol=0.112L=112mL��
��������Ҳ����112mL���壬��2H++2e-�TH2���������������ʵ���Ϊ0.005mol���÷�Ӧת�Ƶĵ���Ϊ0.01mol��
��Cu2++2e-�TCu��ת��0.01mol���ӣ�����Cu2+�����ʵ���Ϊ0.005mol��ͨ��ǰc��CuSO4��=$\frac{0.005mol}{0.2L}$=0.025 mol•L-1��
�ʴ�Ϊ��0.025mol/L��
��4����A�з���2KCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2KOH+H2��+Cl2����2e-���ɵ����غ��֪��ת��0.02mol����ʱ����0.02molKOH��������Һ����ı仯��
��c��OH-��=$\frac{0.02mol}{0.1L}$=0.2mol•L-1��
�ʴ�Ϊ��0.2 mol•L-1��
���� ���⿼����ԭ������ȷCu�缫�����������ǽ�����ͻ�ƿڣ�����ȷ�����ĵ缫��Ӧ�������غ㼴�ɽ��ע�����ʱ�����غ��Ӧ�ã���Ŀ�Ѷ��еȣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 32% | B�� | 24.2% | C�� | 22.2% | D�� | 16.7% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 18g���ľ����к����������ĿΪ2NA | |
| B�� | 11.2 L���飨��״�����к��ЦҼ�����ĿΪ5NA | |
| C�� | 2.0 L 0.5 mol/L NaAlO2��Һ��������ԭ�ӵ���ĿΪ2NA | |
| D�� | 1molCl2������NaOH��Һ��Ӧ��ת�Ƶ��ӵ���ĿΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��� | ��������ĩ״�� ��mol�� | ���Ũ�ȼ���� | ��Ӧ�¶ȣ��棩 |
| A | Mg��0.1 | 5 mol•L-1 ���� 10mL | 60 |
| B | Mg��0.1 | 3 mol•L-1 ���� 10mL | 60 |
| C | Fe��0.1 | 3 mol•L-1 ���� 100mL | 60 |
| D | Mg��0.1 | 2 mol•L-1 ���� 5mL | 60 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{1}{2}$��10-8+10-10��mol/L | B�� | ��10-8+10-10��mol/L | ||
| C�� | ��1��10-4+5��10-10��mol/L | D�� | 2��10-10mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʵ��ٿ�֪��Һ�в�����CO32-��SiO32- | |
| B�� | ����Һ�п϶�����NH4+��Fe2+ | |
| C�� | ����Һ�����ٺ���4������ | |
| D�� | ��ȡ����Һ������������ˮ�͵�����Һ������Һ����ɫ������ȷ������Һ�к���NH4+��Fe2+��SO42-��I- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ƶ�Ӳ�ȴ����� | B�� | þ���۷е���ڸ� | ||
| C�� | þ��Ӳ�ȴ��ڼ� | D�� | �Ƶ��۷е���ڼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NO2 | B�� | FeS | C�� | SO2 | D�� | FeCl3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com