
| ��ѧʽ | CaCO3 | CaSO3 | CaSO4 | CaC2O4 | Mg��OH��2 | MgCO3 |
| KSP | 2.80��10-9 | 4.96��10-9 | 9.01��10-6 | 2.34��10-9 | 1.80��10-11 | 6.82��10-6 |
| 1.8��10-11 |
| (0.01)2 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
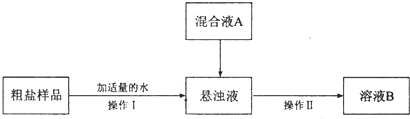
| ����� | �������� | ������ | ��Һ | ���� | ����� |
| ���ڸ�������� | ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
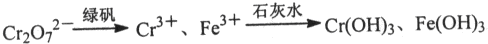
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������1.00mol/L |
| B������1.00mol/L |
| C����1.00mol/L |
| D����ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
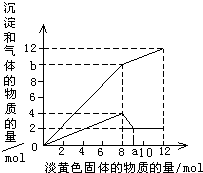 ����һ����Һ�����п��ܺ���Mg2+��Al3+��Fe2+��Cu2+��NH4+��������һ�ֵ���ɫ���岢�����ܽ�ʱ���д̼�����ζ������ų��Ͱ�ɫ�������ɣ����뵭��ɫ��������ʵ����������꣩�������ij����Ͳ�����������ʵ����������꣩�Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
����һ����Һ�����п��ܺ���Mg2+��Al3+��Fe2+��Cu2+��NH4+��������һ�ֵ���ɫ���岢�����ܽ�ʱ���д̼�����ζ������ų��Ͱ�ɫ�������ɣ����뵭��ɫ��������ʵ����������꣩�������ij����Ͳ�����������ʵ����������꣩�Ĺ�ϵ��ͼ��ʾ���Իش��������⣺| ���ܺ��е����� �������ӷ��ţ� |
|||||
| ��Ӧ�����ʵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
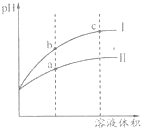 ij�¶��£���ͬpHֵ������ʹ�����Һ�ֱ��ˮϡ�ͣ�pHֵ����Һ����仯��������ͼ��ʾ�������жϲ���ȷ���ǣ�������
ij�¶��£���ͬpHֵ������ʹ�����Һ�ֱ��ˮϡ�ͣ�pHֵ����Һ����仯��������ͼ��ʾ�������жϲ���ȷ���ǣ�������| A������I�������ᣬ���ߢ�������� |
| B����Һ��ˮ�ĵ���̶��ɴ�С��˳��a��b��c |
| C����Һ�ĵ�������ǿ������˳��a��b��c |
| D��a����Һ�м������������ƹ��壬��Һ���Խ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
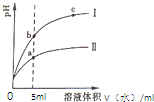 ij�¶��£��ֱ�ϡ�͵�pH������ʹ��ᣬ��ҺpH�����ˮ������仯��������ͼ��ʾ����ͼ�ж�����˵����ȷ���ǣ�������
ij�¶��£��ֱ�ϡ�͵�pH������ʹ��ᣬ��ҺpH�����ˮ������仯��������ͼ��ʾ����ͼ�ж�����˵����ȷ���ǣ�������| A����Ϊ����ϡ��ʱpH�仯���� |
| B����Һ�ĵ����ԣ�b��a��c |
| C��ȡ��0���������������Һ�ֱ���������ۣ��ų�H2������������� |
| D��ȡ5mL�������������Һ�ֱ�����ͬ��Zn����Ӧ����ʼʱ�ķ�Ӧ���ʣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A���ö��Ե缫����Ȼ�þ��Һ��2Cl-+2H+
| ||||
| B������������Һ��Ͷ��Na2O2��SO32-+2Na2O2=SO42-+O2��+4Na+ | ||||
| C��Fe2O3�����������������Һ�У�Fe2O3+6H+=2Fe3++3H2O | ||||
| D�������Ǻʹ�����Һ����Ҫ��Ӧ��CaCO3+2CH3COOH=2CH3COO-+Ca2++H2O+CO2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����pH=7ʱ��һ����c��Na+��=c��CH3COO-��=c��OH-��=c��H+�� |
| B����c�� CH3COO-����c��Na+��ʱ��aһ������12.5 |
| C����a=25ʱ��һ����c��CH3COO��+c��CH3COOH��=c��Na+�� |
| D����c��OH����C��H+��ʱ��aһ��С��12.5 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com