
���ݻ�������ܱ������д������·�Ӧ��2SO2(g)��O2(g) 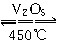 2SO3(g)����H<0��ij�о�С���о���������������ʱ���ı�ijһ������������Ӧ��Ӱ�죬���з�����ȷ����(����)
2SO3(g)����H<0��ij�о�С���о���������������ʱ���ı�ijһ������������Ӧ��Ӱ�죬���з�����ȷ����(����)

A��ͼ���ʾ����t1ʱ������O2��Ũ�ȶԷ�Ӧ���ʵ�Ӱ��
B��ͼ���ʾ����t1ʱ�̼��������Է�Ӧ���ʵ�Ӱ��
C��ͼ���ʾ���Ǵ�����ƽ���Ӱ�죬�ҼĴ�Ч�ʱ��Ҹ�
D��ͼ���ʾ����ѹǿ�Ի�ѧƽ���Ӱ�죬���ҵ�ѹǿ�ϸ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������Ԫ��W��X��Y��Z��ԭ��������������W��Xԭ�ӵ�����������֮��Ϊ4��3��Zԭ�ӱ�Xԭ�ӵĺ����������4������˵����ȷ���ǣ�������
A��W��Y��Z�ĵ縺�Դ�С˳��һ����Z��Y��W
B��W��X��Y��Z��ԭ�Ӱ뾶��С˳�������W��X��Y��Z
C��Y��Z�γɵķ��ӿռ乹�Ϳ�������������
D��WY2�����Цļ���м�����Ŀ֮����2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��̽�����Ʊ�����ˮ����ɺ����ʶ����������¿�ѧʵ�飺�ȹ۲�����ˮ����������Ժ����ý�ͷ�ιܽ�����ˮ��ε��뺬�з�̪��NaOH��Һ�У��ߵα����������۲���������Һ�ĺ�ɫ����ȥ������ɫ��Һ����ش��������⣺
(1)д�����Ʊ�����ˮ�к�����Ԫ�����ʵĻ�ѧʽ��
__________________________________________��
(2)�����ٽ��и����ʵ�飬��˵���ܿ����ж���ˮ�к���Cl2����Ҫ���ݣ�____________________��
(3)����Ԥ�⣬ʵ������Һ��ɫ��ȥ��ԭ����������֣����ü�Ҫ������˵����
��__________________________________________��
��__________________________________________��
(4)ͨ��ʵ���һ��̽����Һ��ɫ��ȥ��ԭ���������еĢٻ��Ǣڡ�
[ʵ�鲽��]
��ȡ�Թ��ڵ���ɫ��Һ3 mLʢ����һ֧�ྻ���Թ��У�
��________________�����Թܡ�
[ʵ��������]
����________________����֤����Һ��ɫ��ȥ��ԭ���Ǣٶ����Ǣڣ�
����________________����֤����Һ��ɫ��ȥ��ԭ���Ǣڶ����Ǣ١�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ӧ����v�ͷ�Ӧ��Ũ�ȵĹ�ϵ����ʵ�鷽���ⶨ�ġ���ѧ��ӦH2��Cl2===2HCl�ķ�Ӧ����v�ɱ�ʾΪv��k[H2]m[Cl2]n��ʽ��kΪ������m��nֵ�����±�������ȷ����
| [H2]/mol��L��1 | [Cl2]/ mol��L��1 | v/mol��L��1��s��1 |
| 1.0 | 1.0 | 1.0 k |
| 2.0 | 1.0 | 2.0 k |
| 2.0 | 4.0 | 4.0 k |
�ɴ˿��Ƶã�m��nֵ��ȷ����(����)
A��m��1��n��1 B��m��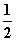 ��n��
��n��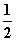
C��m��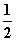 ��n��1 D��m��1��n��
��n��1 D��m��1��n��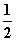
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��Na2S2O3��H2SO4===Na2SO4��SO2����S����H2O�����и���ʵ���У���Ӧ����������(����)
| ��� | ��Ӧ�¶�/�� | Na2S2O3 | H2SO4 | H2O | ||
| ���/ mL | Ũ��/ mol��L��1 | ���/ mL | Ũ��/ mol��L��1 | ���/ mL | ||
| A | 10 | 5 | 0.2 | 5 | 0.1 | 10 |
| B | 10 | 5 | 0.1 | 5 | 0.1 | 10 |
| C | 30 | 5 | 0.1 | 5 | 0.1 | 10 |
| D | 30 | 5 | 0.2 | 5 | 0.2 | 10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ����ϡ������п��ȡ������ʵ���У����ּ�����������ͭ��Һ�ɼӿ��������������ʡ���ش��������⣺
(1)����ʵ���з�����Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
(2) ����ͭ��Һ���Լӿ������������ʵ�ԭ����
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
(3)ʵ����������Na2SO4��MgSO4��Ag2SO4��K2SO4��4����Һ����������ʵ����CuSO4��Һ���������õ���________��
(4)Ҫ�ӿ�����ʵ����������������ʣ����ɲ�ȡ�Ĵ�ʩ��
________________________________________________________________________
_______________________________________________________________(������)��
(5)Ϊ�˽�һ���о�����ͭ�����������������ʵ�Ӱ�죬��ͬѧ���������һϵ�е�ʵ�顣�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���ķ�Ӧƿ�У��ռ����������壬��¼�����ͬ��������������ʱ�䡣
| ʵ�� �����Һ������ | A | B | C | D | E | F |
| 4 mol��L��1H2SO4/mL | 30 | V1 | V2 | V3 | V4 | V5 |
| ����CuSO4��Һ/mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
| H2O/mL | V7 | V8 | V9 | V10 | 10 | 0 |
������ɴ�ʵ����ƣ����У�V1��________��V6��________��V9��________��
�ڷ�Ӧһ��ʱ���ʵ��A�еĽ�����________ɫ��ʵ��E�еĽ�����________ɫ��
�۸�ͬѧ���ó��Ľ���Ϊ������������CuSO4��Һʱ���������������ʻ�����ߣ����������CuSO4��Һ����һ����ʱ���������������ʷ������½���������������������½�����Ҫԭ��
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�¶��£������Ϊ2 L���ܱ������г���1 mol A��b mol B���壬�������·�Ӧ��A(g)��B(g)
 2C(g)��5 min��Ӧ��ƽ��ʱn(A)Ϊ0.4 mol���ڷ�Ӧ��������ϵ���¶ȳ������ߣ�ʵ���û��������C�ĺ������¶ȵĹ�ϵ����ͼ��ʾ������������ȷ����(����)
2C(g)��5 min��Ӧ��ƽ��ʱn(A)Ϊ0.4 mol���ڷ�Ӧ��������ϵ���¶ȳ������ߣ�ʵ���û��������C�ĺ������¶ȵĹ�ϵ����ͼ��ʾ������������ȷ����(����)
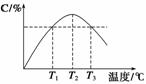
A��0��5 min��C���ʵ�ƽ����Ӧ����Ϊ0.04 mol��L��1��min��1
B��ͼ���¶�T1ʱ������Ӧ���ʵ����¶�T3ʱ������Ӧ����
C���÷�Ӧ�¶�T2ʱ��ƽ�ⳣ�������¶�T3ʱ��ƽ�ⳣ��
D��ͼ��T2ʱ����ֻ����ѹǿ���������淴Ӧ���ʲ��ı�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C�������أ�A����ʢ��CuCl2��Һ����ͭƬ��������B��C�����ھ�ʢ��AgNO3��Һ������˿����������B������˿��������0.108 g��C������˿��������0.216 gʱ��A����ͭƬ��������(����)
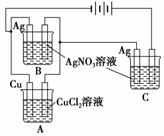
A��0.216 g�������������� B��0.108 g
C��0.064 g D��0.032 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ֱ���ŷź�SO2���������γ����꣬Σ�������������Ƽ�ѭ�������ѳ������е�SO2��
(1)����Һ����SO2�Ĺ����У�pH��n(SO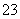 )��n(HSO
)��n(HSO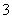 )�仯��ϵ���±���
)�仯��ϵ���±���
| n(SO | 91��9 | 1��1 | 9��91 |
| pH | 8.2 | 7.2 | 6.2 |
���ϱ��жϣ�NaHSO3��Һ��________�ԣ��û�ѧƽ��ԭ�����ͣ�________________________________________________________________________��
(2)������Һ��pH����ԼΪ6ʱ�����͵���������������ʾ��ͼ���£�
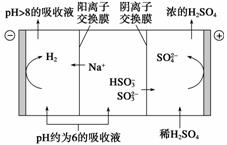
������������ҺpH����8����ʱ������Һ������ѭ�����á���������ԭ����
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com