
ij�л���A������������ܶ�Ϊ43��A����������Ʒ�Ӧ������ɫ���壬������̼���Ʒ�Ӧ������ɫ���壬������ʹ������Ȼ�̼��Һ��ɫ��A��ȫȼ��ֻ����CO2��H2O ��
��1��A����Է�������Ϊ ��A�к��е�2�������ŷֱ��� �� ���ýṹ��ʽ��ʾ����
��2�����˴Ź����ⷢ��A��ͼ�����£�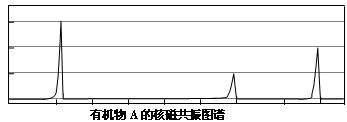
��д��A�Ľṹ��ʽ��________________________________________________��
��3����д��A��״���Ӧ�����л���B�Ļ�ѧ��Ӧ����ʽ��
��
��4��B��һ�������¿��Է����ۺϷ�Ӧ�����л�������д����Ӧ�Ļ�ѧ����ʽ��
��
��5��B��ͬ���칹���ж��֣���д�����ֺ�Bͬ����ͬ���칹��Ľṹ��ʽ ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���1����д���ǻ��ֱ������л������Ϲ������ʵ����ƣ�
�١�CH2CH2�� ��
 ��CH3�� �� ��
��CH3�� �� ��
��2������ ij�л���A���仯ѧʽΪCxHyOz�����ĺ������չ��ױ������ǻ�O-H����������C-H���ĺ������շ壬���������ǻ�����ԭ�Ӹ�����Ϊ2:1��������Է�������Ϊ62������ṹ��ʽΪ ��
�� ij�л���B������������ܶ�Ϊ29��ȼ��2.9�˸��л������3.36��CO2���壬��B�ķ���ʽΪ ��ȡ0.58��B������������Һ��Ӧ������������2.16�ˣ���B�Ľṹ��ʽΪ ��д��B������������ͭ���ȵĻ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡâ����ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��12�֣���1����д���ǻ��ֱ������л������Ϲ������ʵ����ƣ�
�١�CH2CH2�� �� ��CH3�� �� ��
��CH3�� �� ��
��2������ ij�л���A���仯ѧʽΪCxHyOz�����ĺ������չ��ױ������ǻ�O-H����������C-H���ĺ������շ壬���������ǻ�����ԭ�Ӹ�����Ϊ2:1��������Է�������Ϊ62������ṹ��ʽΪ ��
�� ij�л���B������������ܶ�Ϊ29��ȼ��2.9�˸��л������3.36��CO2���壬��B�ķ���ʽΪ ��ȡ0.58��B������������Һ��Ӧ������������2.16�ˣ���B�Ľṹ��ʽΪ ��д��B������������ͭ���ȵĻ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡâ�и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��12�֣���1����д���ǻ��ֱ������л������Ϲ������ʵ����ƣ�
�١�CH2CH2�� ��
 ��CH3�� �� ��
��CH3�� �� ��
��2������ ij�л���A���仯ѧʽΪCxHyOz�����ĺ������չ��ױ������ǻ�O-H����������C-H���ĺ������շ壬���������ǻ�����ԭ�Ӹ�����Ϊ2:1��������Է�������Ϊ62������ṹ��ʽΪ ��
�� ij�л���B������������ܶ�Ϊ29��ȼ��2.9�˸��л������3.36��CO2���壬��B�ķ���ʽΪ ��ȡ0.58��B������������Һ��Ӧ������������2.16�ˣ���B�Ľṹ��ʽΪ ��д��B������������ͭ���ȵĻ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)ij���������һ����̬������һ����̬ϩ����ɣ���ͬ��ͬѹ�£�����������������
���ܶ�Ϊ13���ڱ�״���£���56.0L�������ͨ��������ˮ����ˮ��������35.0g����
�����ṹʽΪ ______ ��������������ϩ�����ʵ���֮��Ϊ_______________��
��2��ij�л���A����Է�������Ϊ62��Ϊ��һ���ⶨA�Ļ�ѧʽ����ȡ6.2 g A��ȫȼ�գ�
�õ�������̼��ˮ�������������Ⱥ�ͨ��������Ũ����ͼ�ʯ�ң����߷ֱ�����5.4 g��
8.8 g (����ÿ����Ӧ����ȫ)�����л����ʵ��ʽ��_____ ������ʽ��____ ____��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com