

 (6��)��1��N ��2��
(6��)��1��N ��2��
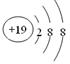
 �������ӵĽṹʾ��ͼ��
�������ӵĽṹʾ��ͼ�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| Ԫ�� | �����Ϣ |
| X | Xԭ�Ӻ��������������Ǵ�����2�� |
| Y | Y����̬�⻯���ˮ��Һ�������� |
| Z | Z�ǵؿ��к������Ľ���Ԫ�� |
| W | ���³�ѹ�£�W�ĵ����ǵ���ɫ���� |
| Q | ���� |
 (1)Ԫ��Q��Ԫ�����ڱ��е�λ��______________________________��
(1)Ԫ��Q��Ԫ�����ڱ��е�λ��______________________________��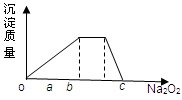
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �е�һ�ֻ������ӣ�ȡ��Һ��������ʵ�飬��ʵ������ת����
�е�һ�ֻ������ӣ�ȡ��Һ��������ʵ�飬��ʵ������ת����
 ��������Ϳ��а㣬����Լ�����ǣ�______________��NaOH��Һ���ڷ�̪�Լ�����ʯ���Լ�����pH��ֽ��KSCN��Һ����KMnO4��Һ��
��������Ϳ��а㣬����Լ�����ǣ�______________��NaOH��Һ���ڷ�̪�Լ�����ʯ���Լ�����pH��ֽ��KSCN��Һ����KMnO4��Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 þ����Ҫ�������£�
þ����Ҫ�������£�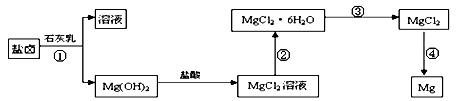
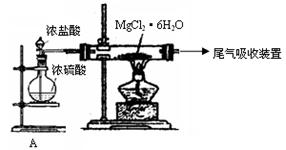
 �ش��������⣺
�ش��������⣺ ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 E�������ӽṹʾ��ͼ�� (1��)
E�������ӽṹʾ��ͼ�� (1��) ������ͨ��1L
������ͨ��1L  1mol��L-1
1mol��L-1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com