
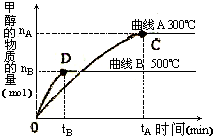
 ��1�������Ϊ3L���ܱ������м���һ������CO��H2��������һ�������·�Ӧ���ɼ״���CO��g��+2H2��g����CH3OH��g������ͬ�¶������£���Ӧ�ӿ�ʼ��ƽ�⣬�״������ʵ����仯��ͼ��ʾ����÷�Ӧ��
��1�������Ϊ3L���ܱ������м���һ������CO��H2��������һ�������·�Ӧ���ɼ״���CO��g��+2H2��g����CH3OH��g������ͬ�¶������£���Ӧ�ӿ�ʼ��ƽ�⣬�״������ʵ����仯��ͼ��ʾ����÷�Ӧ��| ��c |
| ��t |
|
| ||
| tB min |
| nB |
| 3tB |
| nB |
| 3tB |
| 2nB |
| 3tB |
| 2nB |
| 3tB |
|
|


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
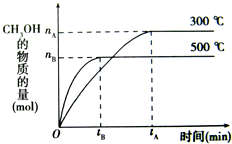 һ�������£������Ϊ3L���ܱ������л�ѧ��ӦCO��g��+2H2��g��-CH3OH��g���ﵽƽ��״̬��
һ�������£������Ϊ3L���ܱ������л�ѧ��ӦCO��g��+2H2��g��-CH3OH��g���ﵽƽ��״̬��| c(CH3OH) |
| c(CO)��c2(H2) |
| c(CH3OH) |
| c(CO)��c2(H2) |
| nB |
| 3tB |
| nB |
| 3tB |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 һ�������£������Ϊ3L���ܱ������л�ѧ��ӦCO��g��+2H2��g��?CH3OH��g���ﵽƽ��״̬��
һ�������£������Ϊ3L���ܱ������л�ѧ��ӦCO��g��+2H2��g��?CH3OH��g���ﵽƽ��״̬��| c(CH3OH) |
| c(CO)��c2(H2) |
| c(CH3OH) |
| c(CO)��c2(H2) |
| ||
| tB |
| ||
| tB |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
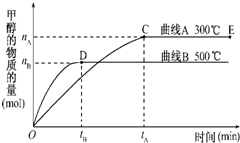 һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO��
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO��| c(CH3OH) |
| c(CO)c2(H2) |
| c(CH3OH) |
| c(CO)c2(H2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��?CH3OH��g��
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��?CH3OH��g��| c(CH3OH) |
| c(CO)?c2(H2) |
| c(CH3OH) |
| c(CO)?c2(H2) |
| 2nB |
| 3tB |
| 2nB |
| 3tB |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com