
����Ŀ����֪��![]()
������ͼװ�����������ϳ�����ȩ![]() ������������
������������
���� | �е� | �ܶ� | ˮ���ܽ��� |
������ |
|
| �� |
����ȩ |
|
| �� |
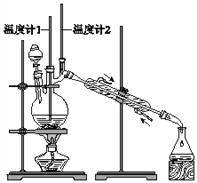
����˵���У�����ȷ����![]()
A. Ϊ��ֹ�����һ��������Ӧ���ữ��![]() ��Һ��μ�����������
��Һ��μ�����������
B. ���¶ȼ�1ʾ��Ϊ![]() ���¶ȼ�2ʾ����
���¶ȼ�2ʾ����![]() ����ʱ���ռ�����
����ʱ���ռ�����
C. ��Ӧ������������ﵹ���Һ©���У���ȥˮ�㣬������ȩ�ӷ�Һ©���Ͽڵ���
D. ���õĴ�����ȩ�м����������ƺ������ɽ�һ����ȥ�ֲ�Ʒ�е�����������
���𰸡�D
��������A��Na2Cr2O7����������������������������Ҳ���ܹ���������������ȩ��Ϊ��ֹ�����һ��������Ӧ�ý��ữ��Na2Cr2O7��Һ��μ����������У���A��ȷ��B���ɷ�Ӧ��Ͳ���ķе����ݿ�֪���¶ȼ�1������90��95�棬�ȿɱ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ���������¶ȼ�2ʾ����76������ʱ���ռ�����Ϊ����ȩ����B��ȷ��C������ȩ�ܶ�Ϊ0.8017 gcm-3��С��ˮ���ܶȣ��ʴ�����ȩ�ӷ�Һ©���Ͽڵ�������C��ȷ��D������������������������ȩ����������Ӧ�����õĴ�����ȩ�м����������ƺ������Գ�ȥ�ֲ�Ʒ�е�������������Ӱ�죬��D����ѡD��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��д���������ʵĻ�ѧʽ��
������_______________��
��С�մ�________________��
��2��д��þ���ڶ�����̼������ȼ�յ���ѧ����ʽ__________________________________________��
��3��д��ʵ�������������ռ���Һ������������������ʽ_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ���ܶ�Ϊ1.84g/cm3����������Ϊ 98%��Ũ��������500mL0.22 mol/L��ϡ���ᡣ
(1)����������ʹ�õ��IJ���������_____________________________________________________��
(2)ʵ��������漰�IJ������£�
�ٽ�����ƿ�е���Һ�����Լ�ƿ�в����ϱ�ǩ��
�ڽ�Ũ�������ձ��ڱڻ���ע��ʢ��Լ160mL����ˮ���ձ��в�С�Ľ��裻
���ù��Ϊ ����Ͳ��ȡ mL��Ũ���
�ܽ�����ȴ��������Һת�Ƶ�����ƿ�У�������������ˮϴ���ձ��Ͳ�����2~3�Σ�ϴ��Һȫ��ת�Ƶ�����ƿ�У�����ҡ������ƿ��ʹ��Һ��Ͼ��ȣ�
�ݸ��ý�ͷ�ιܵμ�����ˮ��ʹ��Һ��Һ����ʹ�ǡ����̶������У�
�����ò������������ߵ�ҡ�ȣ�
����������ƿ�м�����ˮֱ������̶��� ����
����������߲���������_____________��_____________��_______________��
ʵ�����˳��Ϊ__________________________________________(�����)��
(3)���Ƹ���Һ�Ĺ����У����в���ʹ������õ���ҺŨ��ƫС����______________(����ĸ) ��
A��ϡ���ܶ�Ϊ1.84g/cm3����������Ϊ 98%��Ũ����ʱ����С�Ľ�������������Һ
B��������ˮϴ���ձ�����Ͳ��������������ϴ��Һע������ƿ��
C������ʱ�������µ�ת����ƿ���ְ�Һ����ʹ����ڿ̶��ߣ��ٲ���ˮ���̶���
D����ȡ�ܶ�Ϊ1.84g/cm3����������Ϊ 98%��Ũ����ʱ��������Ͳ�Ŀ̶���
E��ϴ������ƿ����������������Һ
F��ϡ��Ũ�����������������Һת�Ƶ�����ƿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��黯�����У����۶������κα�����ϣ�ֻҪ������һ��������ȫȼ��ʱ����O2������������ˮ������������ǣ� ��
A��CH4��C2H6 B��C2H6��C3H6 C��C2H4��C3H6 D��C2H4��C3H4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�Ũ�Ⱦ�Ϊ0.1mol/L�������ΪV0��NaX��NaY��Һ�ֱ��ˮϡ�������V����֪pOH=-lgc(OH-)��pOH��lg(V/V0)�ı仯��ϵ��ͼ��ʾ������˵����ȷ����
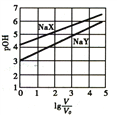
A. Kh(NaY)=10-6
B. HX��HY�������ᣬ��Ka(HX)<Ka(HY)
C. lg(V/V0)=3ʱ��NaX��Һ������������������NaY��Һ
D. ��ϡ��ǰ������Һ�зֱ��������pH=7ʱ��c(X-)=c(Y-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(22Ti)����(26Fe)������ػ������ڻ�����ҽҩ�����ϵ��������Ź㷺��Ӧ�á��ش���������:
(1)��̬Tiԭ���У�����ܲ���ӵĵ�����������״Ϊ_____________����Tiͬ���ڵ����й���Ԫ�صĻ�̬ԭ���У��������������Ѳ�ͬ��Ԫ����_________�֡�
(2)����������Ƭ������ȱ����ƶѪ��Ԥ�������Ƶij���ҩ��ٴ��������ά����C�ٽ��������������գ���������Fe3+���ӽṹ�Ƕ�������Fe2+�ױ�������Fe3+��ԭ����_____________��
(3)SCN-���ӿ�����Fe3+�ļ��飬���Ӧ���������֣��ֱ�Ϊ������(H-S-C![]() N)����������(H-N=C=S)��
N)����������(H-N=C=S)��
��д����SCN-��Ϊ�ȵ������һ����______________(���ӻ�����)��
������������Цм��ͦҼ��ĸ���֮��Ϊ______________��
����������ķе��������е�ߵ�ԭ����__________________________________��
(4)TiCl3������ϩ������ۺϵĴ�������:nCH3CH=CH2![]() ���÷�Ӧ�漰��������̼ԭ�ӵ��ӻ����������_______________________����Ӧ�漰��Ԫ���е縺��������_____________________________��
���÷�Ӧ�漰��������̼ԭ�ӵ��ӻ����������_______________________����Ӧ�漰��Ԫ���е縺��������_____________________________��
(5)Ti��ij�������CaO��������γ������εľ����ṹ��ͼ��ʾ(Ti4+λ��������Ķ��㣬Ca2+���������������)���þ����У�Ti4+����Χ________��O2-���ڣ����þ������ܶ�Ϊdg/cm3���������ļ���Ϊ________cm(�ú�NA�Ĵ���ʽ��ʾ)��
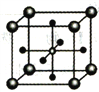
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ������������ء����������г������ʵ������뻯ѧʽ���Ӧ����(����)
A.�����ơ���NaOHB.�̷�����CuSO4��5H2O
C.���ᡪ��C2H5OHD.��ʯ�ҡ���CaSO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ں������������������·�Һ��NH4+��ת��ΪN2(g)��H2O(l)��ʾ��ͼ���£�
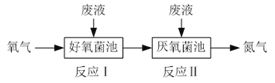
��ӦI��NH4+(aq)��2O2(g)=NO3��(aq)��2H+(aq)��H2O(l) ��H1��a kJ��mol��1
��ӦII��5NH4+(aq)��3NO3��(aq)=4N2(g)��9H2O(l)��2H+(aq) ��H2��b kJ��mol��1
����˵����ȷ����
A. ���ط����ķ�Ӧ�е�Ԫ��ֻ������
B. ������Ͷ�ŵķ�Һ������ʱNH4+����ȫת��ΪN2
C. ���³�ѹ�£���ӦII������22.4 L N2ת�Ƶĵ�����Ϊ3.75��6.02��1023
D. 4NH4+(aq)��3O2(g)=2N2(g)��4H+(aq)��6H2O(l) ��H��![]() (3a��b) kJ��mol��1
(3a��b) kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͨ����ˮ��ɹ�ɵô��Σ����γ�NaCl�⣬������MgCl2��CaCl2��Na2SO4�Լ���ɳ�����ʣ����ξ��Ƶ�ʵ���������¡�����˵������ȷ����

A. �ڵڢٲ���ʹ�ò���������ɼ��ٴ����ܽ�
B. �ڢݲ������ǹ���
C. �ڵڢڢۢܢ�ͨ�����뻯ѧ�Լ����ӣ������Լ�˳��Ϊ��NaOH��Һ��Na2CO3��Һ��BaCl2��Һ��ϡ����
D. ��ȥMgCl2�����ӷ���ʽΪ��Mg2++2OH��Mg(OH)2��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com