
��15�֣�50mL 0.5mol/L��������50mL0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ��㷴Ӧ�ȡ�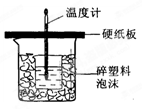
��ش��������⣺
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�� ������ͼ��֪��װ������������֮������1�� ����2 �� ��
��2���ձ���������������ĭ�������ǣߣߣߣߣߣߣߣߡ�
��3�����ձ����粻��Ӳֽ�壬����õķ�Ӧ����ֵ�ߣߣߡ����ƫ����ƫС��������Ӱ�족����
��4����ʵ������У���������NaOH��Һ����ʼ�¶ȵ�ƽ��ֵΪ25.2�森��Һ��Ϻ������¶�Ϊ28.6�森�Ծ�������д����ʾ�÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ��________
�������Һ�ı�����c��4.18J/��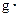 �棩�������NaOH��Һ���ܶ���Ϊ����1
�棩�������NaOH��Һ���ܶ���Ϊ����1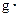
 ��
��
(5)����ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬����к��ȵ���ֵ��ߣߣߣߣ��ƫ����ƫС��������Ӱ�족����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ����кͷ�Ӧ����ѧ��ѧһ����Ҫ�ķ�Ӧ��ijѧ��ʵ��С��Կα��е���������к�ʵ��������о���
����кͷ�Ӧ����ѧ��ѧһ����Ҫ�ķ�Ӧ��ijѧ��ʵ��С��Կα��е���������к�ʵ��������о���| ʵ����� | ��ʼ�ζ��ܶ��� | �յ�ζ��ܶ��� |
| 1 | 0.00mL | 24.02mL |
| 2 | 0.50mL | 24.46mL |
| 3 | 2.50mL | 25.02mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 50mL 0.5mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ���ش��������⣺
50mL 0.5mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com