
Ԫ�ص��ʼ��仯�����й㷺��;����������ڱ��е�������Ԫ�����֪ʶ�ش��������⣺
(1)��ԭ������������˳��(ϡ���������)������˵����ȷ����________��
a��ԭ�Ӱ뾶�����Ӱ뾶����С
b�������Լ������ǽ�������ǿ
c���������Ӧ��ˮ������Լ�����������ǿ
d�����ʵ��۵㽵��
(2)ԭ�������������������������ͬ��Ԫ������Ϊ________�������������ļ���������________��
(3)��֪��
| ������ | MgO | Al2O3 | MgCl2 | AlCl3 |
| ���� | ���ӻ����� | ���ӻ����� | ���ӻ����� | ���ۻ����� |
| �۵�/�� | 2800 | 2050 | 714 | 191 |
��ҵ��þʱ�����MgCl2�������MgO��ԭ����_____ _____________________
_____________________ ______��
______��
����ʱ�����Al2O3�������AlCl3��ԭ����______________________________��
(4)�����(�۵�1410 ��)�����õİ뵼����ϡ��ɴֹ��ƴ���������£�
Si(��)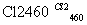 SiCl4
SiCl4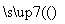 SiCl4(��)
SiCl4(��)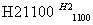 Si(��)
Si(��)
д��SiCl4�ĵ���ʽ��________________����������SiCl4�ƴ���ķ�Ӧ�У����ÿ����1.12 kg����������a kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��_________________________
________________________________________________________________________��
(5)P2O5�Ƿ������Ը�������������岻����Ũ����������P2O5�������________��
a��NH3 ��b��HI c��SO2 d��CO2
(6)KClO3������ʵ������O2�������Ӵ�����400 ��ʱ�ֽ�ֻ���������Σ�����һ�����������Σ���һ���ε��������Ӹ�����Ϊ1��1��д���÷�Ӧ�Ļ�ѧ���� ʽ��________________________________________________________________________��
ʽ��________________________________________________________________________��
 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ķ�Ӧ·��������Ϣ��գ�
A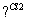
 ��Cl
��Cl 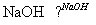

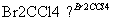 B
B 
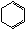
��һ�Ȼ����飩 ��1,3��������ϩ��
(1)A�Ľṹ��ʽ�� ��1mol A��ȫȼ������ mol O2��
(2)��Ӧ�ڵķ�Ӧ������ ��
(3)��Ӧ�ܵĻ�ѧ����ʽ��____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ǽ��ֳ����Ļ�ѧ��Դʾ��ͼ���й�˵������ȷ���� (����)��
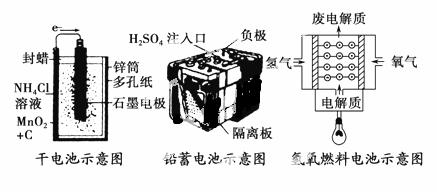
A��������طֱ�����һ�ε�ء����ε�غ�ȼ�ϵ��
B���ɵ���ڳ�ʱ��ʹ�ú�пͲ���ƻ�
C��Ǧ���ع��������У�ÿͨ��2 mol���ӣ�������������207 g
D������ȼ�ϵ����һ�־���Ӧ��ǰ������ɫ��Դ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1��3-����ϩ��2-��Ȳ�ֱ���������Ӧ���Ȼ�ѧ����ʽ���£�
CH2=CH—CH=CH2(g) + 2H2(g) �� CH3CH2CH2CH3(g) + 236.6 kJ
CH3-C��C-CH3(g) + 2H2(g) �� CH3CH2CH2CH3(g) + 272.7 kJ
�ɴ˲����ж�
A��1��3-����ϩ��2-��Ȳ�ȶ��Ե���Դ�С
B��1��3-����ϩ��2-��Ȳ���Ӵ�����������Ըߵ�
C��1��3-����ϩ��2-��Ȳ�ת������ЧӦ
D��һ��̼̼�����ļ���������̼̼˫������֮�͵Ĵ�С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NH3��һϵ�з�Ӧ���Եõ�HNO3������ͼ��ʾ��

��1��I�У�NH3 ��O2�ڴ��������·�Ӧ���仯ѧ����ʽ��_____________________��
��2��II�У�2NO��g��+O2 2NO2(g)��������������ͬʱ���ֱ���NO��ƽ��ת�����ڲ�ͬѹǿ��P1��P2�����¶ȱ仯�����ߣ�����ͼ����
2NO2(g)��������������ͬʱ���ֱ���NO��ƽ��ת�����ڲ�ͬѹǿ��P1��P2�����¶ȱ仯�����ߣ�����ͼ����
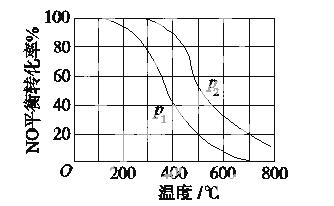
�ٱȽ�P1��P2�Ĵ�С��ϵ��________________��
�����¶����ߣ��÷�Ӧƽ�ⳣ���仯��������________________��
��3��III�У���NO2��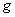 ��ת��ΪN2
��ת��ΪN2 O4��
O4�� �������Ʊ�Ũ���ᡣ
�������Ʊ�Ũ���ᡣ
����֪��2NO2��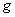 ��
�� N2O4��
N2O4��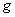 ����H1
����H1
2NO2��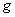 ��
�� N2O4��
N2O4�� �� ��H2
�� ��H2
���������仯ʾ��ͼ�У���ȷ���ǣ�ѡ����ĸ��_______________��
 ��N2O4��O2��H2O���ϵĻ�ѧ����ʽ��___
��N2O4��O2��H2O���ϵĻ�ѧ����ʽ��___ ______________��
______________��
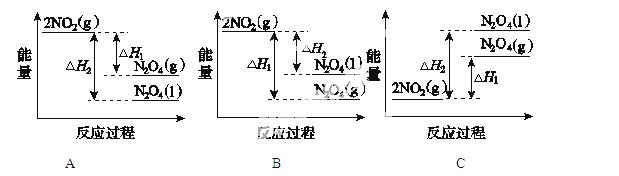
��4��IV�У����NO�Ʊ� NH4NO3���乤��ԭ������ͼ��ʾ��Ϊʹ������ȫ��ת��ΪNH4NO3���貹������A��A��_____________��˵�����ɣ�________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��2Zn(s)��O2(g)===2ZnO(s)����H����701.0 kJ·mol��1 2Hg(l)��O2(g)== =2HgO(s)����H����181.6 kJ·mol��1��ӦZn(s)��HgO(s)===ZnO(s)��Hg(l)�Ħ�HΪ(����)
=2HgO(s)����H����181.6 kJ·mol��1��ӦZn(s)��HgO(s)===ZnO(s)��Hg(l)�Ħ�HΪ(����)
A����519.4 kJ·mol��1���� B����259.7 kJ·mol��1
C����259.7 kJ·mol��1���� D����519.4 kJ·mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
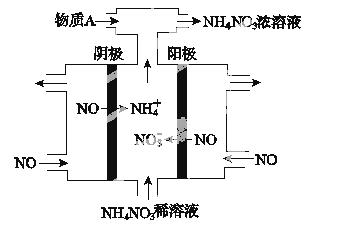
(1)�ٸ�������ͼʾ��д����Ӧ���Ȼ�ѧ����ʽ��________________________________��
�ڸ�����ͼ��ʾ������ж�����˵������ȷ����_________________________________
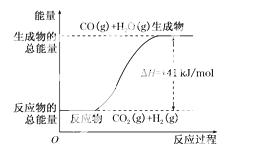
A�����Ȼ�ѧ����ʽΪ��CO(g)��H2O(g)===CO2(g)��H2(g)����H����41 kJ/mol
B���÷�ӦΪ���ȷ�Ӧ
C���÷�ӦΪ���ȷ�Ӧ
D����H2OΪҺ̬ʱ���䷴Ӧ��ֵС��41 kJ/mol
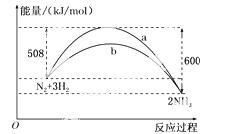
(2)��֪16 g��������ȫȼ��ʱ�ų�148.4 kJ��������
�÷�Ӧ���Ȼ�ѧ����ʽ��_________________________________________________ _��
_��
(3)��ͼ��ij�¶��£�N2��H2��Ӧ�����������仯������ͼ���÷�Ӧ���Ȼ�ѧ����ʽΪ��
______ ____________________________________________��
____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȷ��ʾ���з�Ӧ�����ӷ�Ӧ����ʽΪ�� ��
A��NH4HCO3���ڹ�����ŨKOH��Һ�У�NH4++ HCO3-+2OH-= CO32-+ NH3��+2 H2O
B����������Һ�еμ�Ba(OH)2��Һ��ǡ��ʹSO42-������ȫ��
2Al3++3SO42-+3Ba2++6OH -=2 Al(OH)3��+3BaSO4��
C����FeBr2��Һ��ͨ������������2Fe2++4Br-+3Cl2=2 Fe3++2 Br2+6 Cl-
D�������ȥˮ����2H++CaCO3=Ca2++ CO2��+ H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ�У����ձ���ʢ��100 mL 0.50 mol·L��1 AgNO3��Һ�����ձ���ʢ��100 mL 0.25 mol·L��1 CuCl2��Һ��A��B��C��D��Ϊ������ͬ��ʯī�缫���� �����һ��ʱ�����A����C����1.9 g����
�����һ��ʱ�����A����C����1.9 g����
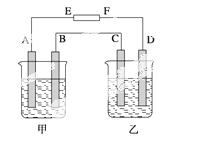
(1)��ԴEΪ________����FΪ________����
(2)A���ĵ缫��ӦʽΪ_____________________________________________��
��������______  mol��
mol��
(3)B���ĵ缫��ӦʽΪ________________________________________________��
��������________ mL(��״��)��
(4)C���ĵ缫��ӦʽΪ_____________________________________________��
����������________ mol��
(5)D���ĵ缫��ӦʽΪ_____________________________________________��
��������________ mL(��״��)��
(6)���ձ��е���ʯ����Һ��________��������죬����������룬�ڼ��ձ������յõ�______��Һ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com