
 ЙъОпЦКДЬКЗТ»ЦЦЅаѕ»ЎўїЙФЩЙъµДДЬФґЈ®ЙъОпЦКЖшЈЁЦчТЄіЙ·ЦОЄCOЎўCO2ЎўH2µИЈ©УлH2»мєПЈ¬ґЯ»ЇєПіЙјЧґјКЗЙъОпЦКДЬАыУГµД·Ѕ·ЁЦ®Т»Ј®
ЙъОпЦКДЬКЗТ»ЦЦЅаѕ»ЎўїЙФЩЙъµДДЬФґЈ®ЙъОпЦКЖшЈЁЦчТЄіЙ·ЦОЄCOЎўCO2ЎўH2µИЈ©УлH2»мєПЈ¬ґЯ»ЇєПіЙјЧґјКЗЙъОпЦКДЬАыУГµД·Ѕ·ЁЦ®Т»Ј®| 1 |
| 8 |
| 1 |
| 2 |



| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє

Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2013-2014С§ДкЛДґЁКЎґпЦЭКРёЯИэµЪТ»ґОХп¶ПјмІвАнЧЫ»ЇС§КФѕнЈЁЅвОц°жЈ© МвРНЈєМоїХМв
ЙъОпЦКДЬКЗТ»ЦЦЅаѕ»ЎўїЙФЩЙъДЬФґЎЈЙъОпЦКЖш(ЦчТЄіЙ·ЦОЄ COЎўCO2ЎўH2 µИ)УлH2»мєПЈ¬ґЯ»ЇєПіЙјЧґјєН¶юјЧГСЈЁCH3OCH3Ј©ј°Рн¶аМюАаОпЦКµИЈ¬КЗЙъОпЦКДЬАыУГµД·Ѕ·ЁЦ®Т».
ЈЁ1Ј©ТСЦЄМјµДЖш»Ї·ґУ¦ФЪІ»Н¬ОВ¶ИПВЖЅєвіЈКэµД¶ФКэЦµЈЁlgKЈ©ИзПВ±н:

·ґУ¦ЈєCO(g)+H2O(g) CO2(g)+H2(g)Ј¬ёГ·ґУ¦µДЎчH________0ЈЁСЎМоЈєЎ°ЈѕЎ±ЎўЎ°ЈјЎ±ЎўЎ°ЈЅЎ±Ј©Ј»ФЪ900KК±Ј¬ёГ·ґУ¦ЖЅєвіЈКэµД¶ФКэЦµЈЁlgKЈ©=_____________.
CO2(g)+H2(g)Ј¬ёГ·ґУ¦µДЎчH________0ЈЁСЎМоЈєЎ°ЈѕЎ±ЎўЎ°ЈјЎ±ЎўЎ°ЈЅЎ±Ј©Ј»ФЪ900KК±Ј¬ёГ·ґУ¦ЖЅєвіЈКэµД¶ФКэЦµЈЁlgKЈ©=_____________.
ЈЁ2Ј©јЧґјКЗТ»ЦЦЦШТЄµДДЬФґєН»Ї№¤ФБПЈ¬№¤ТµЙПєПіЙјЧґјµД·ґУ¦ОЄЈєCO+2H2⇌CH3OHЈ®ПЦТСЦЄЈєH2(g)ЎўCO(g)ЎўCH3OH(l)µДИјЙХИИ¦¤H·Ц±рОЄ-285.8KJ/molЎў-283.0KJ/molєН-726.5KJ/molЎЈФтЈєCH3OHІ»НкИ«ИјЙХЙъіЙCOєНТєМ¬H2OµДИИ»ЇС§·ґУ¦·ЅіМКЅ .
ЈЁ3Ј©ФЪТ»¶ЁОВ¶ИЎўС№ЗїєНґЯ»ЇМхјюПВЈ¬№¤ТµЙПУГCOєНH2·ґУ¦ЙъіЙ¶юјЧГСЈ¬Н¬К±ІъЙъТ»ЦЦІОУлґуЖшС»·µДОЮ»ъОпЎЈФтёГ·ґУ¦µД»ЇС§·ґУ¦·ЅіМКЅОЄЈє Ј®
ЈЁ4Ј©ПВНјЧуОЄВМЙ«µзФґЎ°¶юјЧГСИјБПµзіШЎ±µД№¤ЧчФАнКѕТвНјЈ®aµзј«ЙП·ўЙъ·ґУ¦µДµзј«·ґУ¦КЅОЄ .

ЈЁ5Ј©Б¬ЅУПВНјУТЧ°ЦГµДµзФґОЄЈЁ4Ј©ОКЦРµД¶юјЧГСИјБПµзіШЎЈЅУНЁµзФґТ»¶ОК±јдєуЈ¬№ЫІмµЅЧ°ЦГЦРµзЅвЦКИЬТєСХЙ«УЙОЮЙ«±дОЄА¶Й«Ј¬ІўЦрЅҐјУЙоЎЈФтёГЧ°ЦГЦРµДCuµзј«У¦Ул¶юјЧГСИјБПµзіШЦР µзј«ЈЁМоa»тbЈ©ПаБ¬ЎЈНЁµзК±·ўЙъ·ґУ¦µДЧЬµДАлЧУ·ґУ¦·ЅіМКЅОЄЈє .
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2012-2013С§ДкЛДґЁКЎµВСфКРЎ°Т»ХпЎ±їјКФ»ЇС§КФѕнЈЁЅвОц°жЈ© МвРНЈєМоїХМв
ЙъОпЦКДЬКЗТ»ЦЦЅаѕ»ЎўїЙФЩЙъДЬФґЎЈЙъОпЦКЖшЈЁЦчТЄіЙ·ЦОЄCOЎўCO2ЎўH2µИЈ©Ул»мєПЈ¬ФЪє¬УРZnЎўCuµИФЄЛШµДґЯ»ЇјБМхјюПВДЬєПіЙТ»ЦЦЙъОпЦКДЬЎЄЎЄјЧґјЎЈ
ЈЁ1Ј© УлCO»ҐОЄµИµзЧУМеµДОпЦКµД»ЇС§КЅКЗ ЎЈ
ЈЁ2Ј© CO2·ЦЧУЦРМјФЧУµДФУ»ЇАаРНКЗ ФУ»ЇЎЈ
ЈЁ3Ј© °ґµзЧУЕЕІјZnФЪФЄЛШЦЬЖЪ±нЦРКфУЪ ЗшЈ¬
Ждѕ§МеКфУЪБщ·ЅЧоГЬ¶С»эЈ¬ЛьµДЕдО»КэКЗ ЎЈ
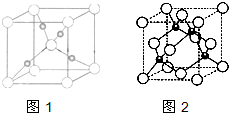
ЈЁ4Ј© CuµДТ»ЦЦВИ»ЇОпѕ§МеµДѕ§°ыЅб№№ИзНјЛщКѕЎЈЈЁїХРДЗтґъ±нВИАлЧУЈ©Ј¬ФтТ»ёцѕ§°ыЦРЛщє¬µДКэДїКЗ ЎЈ

Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2014ЅмЛДґЁКЎёЯ¶ю10ФВЅЧ¶ОРФїјКФ»ЇС§КФѕнЈЁЅвОц°жЈ© МвРНЈєМоїХМв
(5·Ц)ЙъОпЦКДЬКЗТ»ЦЦЅаѕ»ЎўїЙФЩЙъДЬФґЎЈЙъОпЦКЖш(ЦчТЄіЙ·ЦОЄ COЎўCO2ЎўH2 µИ)Ул H2 »мєПЈ¬ґЯ»ЇєПіЙјЧґјКЗЙъОпЦКДЬАыУГµД·Ѕ·ЁЦ®Т»ЎЈ
(1)ЙПКц·ґУ¦µДґЯ»ЇјБє¬УР CuЎўZnЎўAl µИФЄЛШЎЈРґіц»щМ¬ Cu2+АлЧУµДєЛНвµзЧУЕЕІјКЅ_______________________________________;
(2)ёщѕЭµИµзЧУФАнЈ¬Рґіц CO ·ЦЧУµДЅб№№КЅ______________________;
(3)јЧґјґЯ»ЇСх»ЇїЙµГµЅјЧИ©Ј¬јЧИ©УлРВЦЖ Cu(OH)2 µДјоРФИЬТє·ґУ¦ЙъіЙ Cu2O іБµнЎЈ
ўЩјЧИ©·ЦЧУЦРМјФЧУ№мµАµДФУ»ЇАаРНОЄ_____________________;
ўЪјЧИ©·ЦЧУµДїХјд№№РНКЗ__________________Ј»
ўЫ 1 mol јЧИ©·ЦЧУЦР ¦Т јьµДКэДїОЄ__________________ЎЈ
Ійїґґр°ёєНЅвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com