
| A��0�桫50�� | B��0�桫100�� | C��0�桫200�� | D��0�桫360�� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
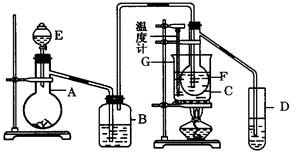
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
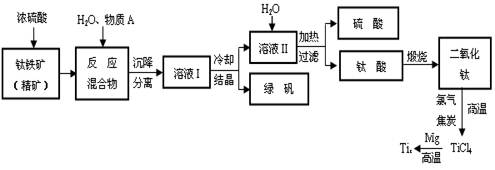
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
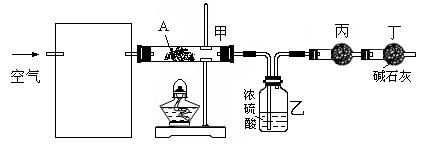
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
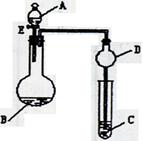
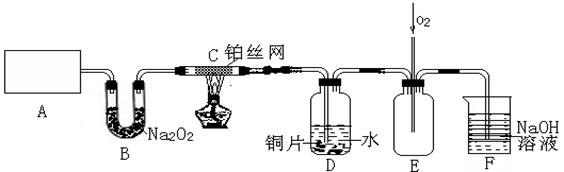 ��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ___________________________��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�___________�������и�����ţ�
��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ___________________________��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е�___________�������и�����ţ�| A��Na2CO3 | B��AgNO3 | C��H2SO4 | D��FeSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
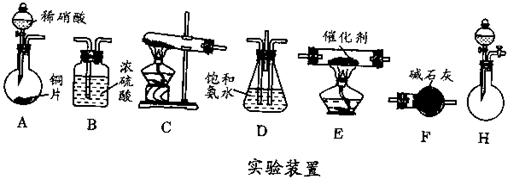
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��aΪϡ���� |
| B��bΪ���Ȼ�̼ |
| C�������պ����Һ������Ϊ��һ����ʱ����һ�������ԡ� |
| D�������չ��̣���������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 _____________��
_____________��
| �Լ������� | �����Լ�/g | NH3���/mL |
| a | 12.0g Ca(OH)2(����) 10.8g NH4Cl | 2688 |
| b | 12.0g Ca (OH)2(����) 10.8g(NH4)2SO4 (OH)2(����) 10.8g(NH4)2SO4 | 2728 |
| c | 12.0g NaOH(����) 10.8g NH4Cl | 3136 |
| d | 12.0g NaOH(����) 10.8g (NH4)2SO4 | 3118 |
| e | 12.0g CaO(����) 10.8g NH4Cl | 3506 |
| f | 12.0g CaO(����) 10.8g (NH4)2SO4 | 3584 |
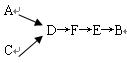
 ____________��
____________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
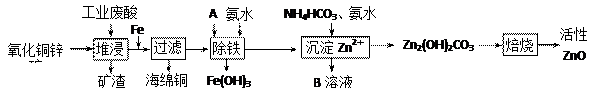
| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2�� | 6.34 | 9.7 |
| Fe3�� | 1.48 | 3.2 |
| Zn2�� | 6.2 | 8.0 |
| A��KMnO4 | B��O2 | C��H2O2 | D��Cl2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com