
��1���õ���ʽ��ʾH2O��MgBr2���γɹ���
H2O MgBr2
��2��д��CO2 ��Na2O2��H2O2�ĵ���ʽ��
CO2 Na2O2 H2O2
��3�� H2O�� ����ϣ�MgBr2�� ����ϡ�NaOH�� ����ϣ�Na2O2�� ����ϣ���Լ����Ǽ��Լ������Ӽ���
��4��![]() ��
��![]() ��
��![]() ��D��E 5�����ӣ����ӻ����ӣ������Ƿֱ�10�����ӣ���֪����������ת����ϵ����
��D��E 5�����ӣ����ӻ����ӣ������Ƿֱ�10�����ӣ���֪����������ת����ϵ����![]() ����
����![]() ��
��
�ݴˣ��ش��������⣺
��д���ٷ�Ӧ�����ӷ���ʽ ��
��![]() ��C-�ĵ���ʽ
��C-�ĵ���ʽ![]() ____________��C-____________��
____________��C-____________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�� ���� |
��A | ��A | ��A | IVA | VA | VIA | VIIA | 0�� |
| 1 | ||||||||
| 2 | G | H | D | |||||
| 3 | B | C | E | |||||
| 4 | F | A |


| Ԫ�� | ʵ����� | ���� | ���� |
| B | �����ԣ�B �� �� F�����������������=���� | ||
| F |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�� ���� |
I A | II A | III A | IV A | V A | VI A | VII A | 0 |
| 2 | E | F | ||||||
| 3 | A | C | D | G | R | |||
| 4 | B | H |
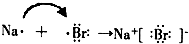
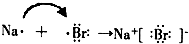
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
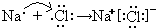
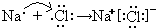
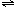 NH3?H2O+H+
NH3?H2O+H+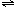 NH3?H2O+H+
NH3?H2O+H+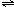 BiOCl+2HCl
BiOCl+2HCl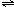 BiOCl+2HCl
BiOCl+2HCl�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ӧ�٢�����Ҫ�Ļ�����Ӧ��D��E��F��G��MΪ���ʣ�D��E��G��HΪ���壬��ֻ��EΪ��ɫ���壬G�ǿ�������Ҫ�ɷ�֮һ��F��M�������г����Ľ�����K�ǰ�ɫ������C����ɫ��ӦΪ��ɫ��P��M���Ϻ�ɫ���嵥�ʻ��ϵIJ��P��J��L��M����ͬ��Ԫ�أ������ʼ�ת����ϵ���£������������Ƶ����ݣ���Ӧ��ֻ����A��N�е����ʡ���ش��������⣺

��1���õ���ʽ��ʾH���ʵ��γɹ���
��2�����آ��е��������������
��3��������E��P��Һ��Ӧ����J�����ӷ���ʽΪ
��4��N��Һ�ʼ��ԣ������ӷ���ʽ����ԭ��
��5����ģ����������ת����ϵͼ���F��K�����;����(��A��N�е����ʵĻ�ѧʽ��ʾ)
 |
F �� ��K
����Ƶõ�1molK��������Ҫ���ӵ��Լ��������ʵ�����
n( )= mol��n( )= mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ��Ҵ���и�һ��ѧ�����п��Ի�ѧ�Ծ� ���������� ���ͣ������
(28�֣�ÿ��2��)�±���Ԫ�����ڱ��ж�����Ԫ�ص�һ���֣�����������ĸ�ֱ����һ��Ԫ�ء�
 ���� �������� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 1 | A | | | | | | | B |
| 2 | | | | D | E | F | J | |
| 3 | C | | | G | | H | I | |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com