
��֪H2(g)�� O2(g)===H2O(g)����H����241.8 kJ��mol��1������˵���в���ȷ���� �� ��
O2(g)===H2O(g)����H����241.8 kJ��mol��1������˵���в���ȷ���� �� ��
| A��H2��ȼ����Ϊ241.8 kJ��mol��1 |
| B��2H2(g)��O2(g)===2H2O(g)����H����483.6 kJ��mol��1 |
| C��1 mol H2��ȫȼ������Һ̬ˮ�ų�����������241.8 kJ |
| D���Ͽ�1 mol H2O�Ļ�ѧ�����յ����������ڶ���1 mol H2��0.5 mol O2�Ļ�ѧ�������յ������� |
A
�������������ȼ��������һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų�������������ˮ��״̬����̬�������ȶ�״̬��ˮ���ȶ�״̬Ӧ����Һ̬��A����ȷ��B��ȷ��������̬ˮ����������Һ̬ˮ������������1mol H2��ȫȼ������Һ̬ˮ�ų�����������241.8 kJ��C��ȷ����Ӧ�Ⱦ��Ƕϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ������D��ȷ����ѡA��
���㣺����ȼ���ȡ���Ӧ�ȵ��й��жϺͼ���
�������������е��Ѷȵ����⣬Ҳ�Ǹ߿��еij�������֮һ������ע�ػ�����������˫�飬����������ѧ��������������������Ĺؼ�����ȷȼ�����Լ���Ӧ�ȵĸ��Ȼ��������������ü��ɡ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ��������ѧ2011��2012ѧ��߶���ѧ�����е��п��Ի�ѧ���� ���ͣ�013
|
��֪H2(g)�� CO(g)�� ijH2��CO�Ļ��������ȫȼ��ʱ�ų�113.74 kJ������ͬʱ����3.6 gҺ̬ˮ����ԭ���������H2��CO�����ʵ���֮��Ϊ | |
| [����] | |
A�� |
2��1 |
B�� |
1��2 |
C�� |
1��1 |
D�� |
2��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���¿γ�ͬ��ѧ��ר�Ұ������ѧѡ��4(��ѧ��Ӧԭ��)��³�ư� ³�ư� ���ͣ�022
��˹������ָһ����ѧ��Ӧ������һ����ɻ��Ƿּ�����ɣ�________��һ���ģ�����ѧ��Ӧ���ʱ�ֻ�뷴Ӧ��________�йأ����뷴Ӧ��;���أ�
���ø�˹���ɼ��㻯ѧ��Ӧ�ʱ�ķ�������һ����ѧ����ʽ�������⼸����ѧ����ʽ��Ӽ����õ�������Щ��ѧ��Ӧ���ʱ���Ӧ________���������Ҫ����Ļ�ѧ��Ӧ���ʱ䣮
������
(1)��֪��C(s)��O2(g)��CO2(g)����H����393.5 kJ��mol��1��CO(g)��![]() O2(g)��CO2(g)����H����283.0 kJ��mol��1��д��C(s)ȼ������CO���Ȼ�ѧ����ʽ��___________��
O2(g)��CO2(g)����H����283.0 kJ��mol��1��д��C(s)ȼ������CO���Ȼ�ѧ����ʽ��___________��
(2)��֪��C(s)��![]() O2(g)��CO(g)����H����110.5 kJ��mol��1��2H2(g)��O2(g)��2H2O(g)����H����483.6 kJ��mol��1����ӦC(s)��H2O(g)��CO(g)��H2(g)�Ħ�HΪ
O2(g)��CO(g)����H����110.5 kJ��mol��1��2H2(g)��O2(g)��2H2O(g)����H����483.6 kJ��mol��1����ӦC(s)��H2O(g)��CO(g)��H2(g)�Ħ�HΪ
A��262.6 kJ��mol��1
B����131.3 kJ��mol��1
C����352.3 kJ��mol��1
D��131.3 kJ��mol��1
(3)��֪H2(g)��![]() O2(g)��H2O(l)����H����285.83 kJ��mol��1��CO(g)��
O2(g)��H2O(l)����H����285.83 kJ��mol��1��CO(g)��![]() O2(g)��CO2(g)����H����282.9 kJ��mol��1����������һ����̼�Ļ��������ȫȼ�տ�����5.4 gҺ̬ˮ�����ų�114.03 kJ������������������CO�����ʵ���Ϊ
O2(g)��CO2(g)����H����282.9 kJ��mol��1����������һ����̼�Ļ��������ȫȼ�տ�����5.4 gҺ̬ˮ�����ų�114.03 kJ������������������CO�����ʵ���Ϊ
A��0.22 mol����B��0.15 mol����C��0.1 mol����D��0.05 mol
(4)0.3 mol����̬������(B2H6)����ȼ����������ȼ�����ɹ�̬B2O3��Һ̬ˮ�����ų�649.5 kJ���������Ȼ�ѧ����ʽΪ________________________����֪H2O(l)��H2O(g)����H��44 kJ��mol��1����11.2 L��״������������ȫȼ��������̬ˮʱ�ų�������Ϊ________kJ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ѧϰ�ܱ�����ѧ���˽̿α�߶���(ѡ��4)��2009��2010ѧ�ꡡ��1�ڡ��ܵ�157�� �˽̿α��(ѡ��4) ���ͣ�022
��֪
H2(g)��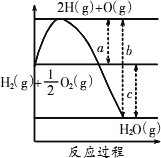
(1)a��b��c�ֱ��������˼��________��________��________��
(2)�÷�Ӧ��________(����ȡ����ȡ�)��Ӧ����H________(���0����0��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭���Ͼ�ѧ�����ר��ѧУ�߶�3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪H2(g)��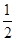 O2(g)===H2O(g)����H����241.8 kJ��mol��1������˵���в���ȷ���� �� ��
O2(g)===H2O(g)����H����241.8 kJ��mol��1������˵���в���ȷ���� �� ��
A��H2��ȼ����Ϊ241.8 kJ��mol��1
B��2H2(g)��O2(g)===2H2O(g)����H����483.6 kJ��mol��1
C��1 mol H2��ȫȼ������Һ̬ˮ�ų�����������241.8 kJ
D���Ͽ�1 mol H2O�Ļ�ѧ�����յ����������ڶ���1 mol H2��0.5 mol O2�Ļ�ѧ�������յ�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com