
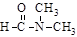 ����ˮ
����ˮ 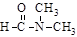 ����ˮ���ļ�ˮ�⣬�ƻ����ǹ��ۼ������������Ȼ�̼�ƻ����Ƿ��Ӽ���������ʯӢ����
����ˮ���ļ�ˮ�⣬�ƻ����ǹ��ۼ������������Ȼ�̼�ƻ����Ƿ��Ӽ���������ʯӢ����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��X��Y | B��X��W | C��Y��Z | D��Y��W |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��4p2��ʾ4p�ܼ���������� |
| B��H2O��NH3��NF3�ļ����������� |
| C��ԭ������Ϊ15��25��35��Ԫ�ض�λ�����ڱ�p�� |
| D��C2H5Cl��H2O2���Ǻ��зǼ��Լ��ļ��Է��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A�� �ų�������һ���Է��Ļ�ѧ�仯���̣����߶���Σ���ܴ� �ų�������һ���Է��Ļ�ѧ�仯���̣����߶���Σ���ܴ� |
| B����ͬԪ�ص�ԭ�ӹ��ɵķ���ֻ�����Թ��ۼ� |
C�� U�� U�� U����������ͬ��������ͬ��ͬ�ֺ��� U����������ͬ��������ͬ��ͬ�ֺ��� |
| D�������ڵڢ�A���A��Ԫ�ص�ԭ�Ӽ乹�ɵķ��ӣ�������ԭ�������8���ӽṹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
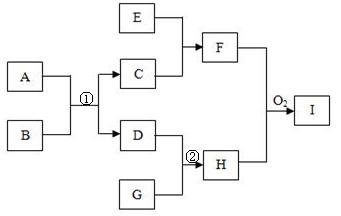
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ������ | H+��Na+��Al3+��Ag+�� Ba2+ |
| ������ | OH���� Cl����CO32����NO3����SO42�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com