
����Ӧ����㷺�Ľ���������±����������Լ������ĺ������ξ�Ϊ��Ҫ�����
(1)Ҫȷ������ij�Ȼ���FeClx�Ļ�ѧʽ���������ӽ����͵ζ��ķ�����ʵ���г�ȡ0.54 g��FeClx��Ʒ���ܽ���Ƚ��������ӽ���Ԥ��������ͨ�����б���OH���������ӽ�������ʹCl����OH������������������ɺ�������Һ��OH����0.40 mol��L��1������ζ��������յ�ʱ��������25.0 mL���������Ʒ���ȵ����ʵ����������FeClx��x��ֵ��__________________(�г��������)��
(2)����һ����FeCl2��FeCl3�Ļ������Ʒ�����������������n(Fe)��n(Cl)��1��2.1�������Ʒ��FeCl3�����ʵ�������Ϊ__________����ʵ�����У�FeCl2�������ۺ�__________��Ӧ�Ʊ���FeCl3�������ۺ�__________��Ӧ�Ʊ���
�𰸡�(1)n(Cl)��0.025 0 L��0.40 mol��L��1��0.010 mol
0��54 g��0.010 mol��35.5 g��mol��1��0.185 g
n(Fe)��0.185 g/56 g��mol��1��0.003 3 mol
n(Fe)��n(Cl)��0.003 3��0.010��1��3��x��3
(2)0.10�����ᡡ����
������������Ĺؼ�����ȷ�����ӽ������� ������OH�������ʵ�������Cl�������ʵ������Ӷ����FeClx��x��ֵ��
������OH�������ʵ�������Cl�������ʵ������Ӷ����FeClx��x��ֵ��
(1)������ȷ�����ӽ������������ӽ����������ã��������ӵ�����������Һ��OH�������ʵ�������FeClx��Cl�������ʵ�����ͨ���к͵ζ�֪n(OH��)��n(H��)��0.40 mol��
L��1��25.0��10��3 L��0.010 mol����n(Cl��)��0.010 mol��
FeClx��FeԪ�ص�����Ϊ0.54 g��35.5 g��mol��1��0.010 mol��0.185 g
FeClx��FeԪ����ClԪ�ص����ʵ���֮��Ϊ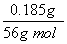 ��0.010 mol��1��3����x��3��
��0.010 mol��1��3����x��3��
(2)�����������û��������ΪFeCl2.1������ʮ�ֽ��淨�ɵ���Ʒ��FeCl3�����ʵ�������Ϊ0.10��ע���Ʊ�FeCl2ѡ�������������Ʊ�FeCl3ѡ��ǿ��������


 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��A��B��C��D��E��F�� G��H��Ϊ�л�������ش��������⣺
G��H��Ϊ�л�������ش��������⣺
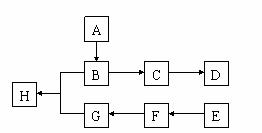
��1���л�������A����Է�������С��60��A�ܷ����۾���Ӧ��1 mol A�ڴ�������������
3 mol H2��Ӧ����B����A�Ľṹ��ʽ�� ����A����B�ķ�Ӧ������
��2��B��Ũ�����м��ȿ�����C��C�ڴ��������¿ɾۺ����ɸ߷��ӻ�����D���ڴ�����������C����D�Ļ�ѧ����ʽ��
��3���ٷ��㻯����E�ķ���ʽ��C8H8Cl2��E�ı����ϵ�һ��ȡ����ֻ��һ�֣���E�����п��ܵ�ͬ���칹�干�� �֡�
��E��NaOH��Һ�п�ת��ΪF��F�ø������������Һ��������G��C8H6O4����1 mol G��������NaHCO3��Һ��Ӧ�ɷų�44.8 L CO2����״�������ɴ�ȷ��E�Ľṹ��ʽ��
��4��G��������B��Ũ������¼��ȷ�Ӧ������H����H��NaOH��Һ�м��ȷ�Ӧ�Ļ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ܱ������е�һ����������巢����Ӧ�� xA(g)+yB(g) zC(g)��ƽ��ʱ���A��Ũ��0.50mol/L�������¶Ȳ��䣬���������ݻ���ԭ�����������ٴ�ƽ��ʱ�����A��Ũ�Ƚ���Ϊ0.30mol/L�������й��жϴ�����ǣ���
zC(g)��ƽ��ʱ���A��Ũ��0.50mol/L�������¶Ȳ��䣬���������ݻ���ԭ�����������ٴ�ƽ��ʱ�����A��Ũ�Ƚ���Ϊ0.30mol/L�������й��жϴ�����ǣ���
A��x+y<z B��ƽ�����淴Ӧ�����ƶ� C��B��ת���ʽ��� D��C����������½�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ������Fe��FeO��Fe2O3��Fe3O4�Ļ�����м���150 mL 4 mol��L��1��ϡ����ǡ��ʹ�������ȫ�ܽ⣬�ų�2.24 L NO(��״��)����������Һ�м���KSCN��Һ����Ѫ��ɫ���֡�����������H2�ڼ��������»�ԭ��ͬ�����Ļ������õ����������ʵ���Ϊ
(����)
A��0.21 mol B��0.25 mol
C��0.3 mol D��0.35 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ����֤Fe3�������ʣ�ij��ѧ��ȤС���������ͼ��ʾ��һ��ʵ�飬����ʵ�鷽����ƴ������ (����)
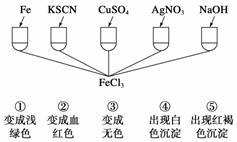
A���� B���� C���ۢ� D���٢ڢۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���� (����)
A��Fe��һ����������ˮ��Ӧ����H2��Fe(OH)3
B��Fe3O4�����������뼸��KSCN��Һ����Һ��Ѫ��ɫ
C��FeCl2��Һ�������պ�õ�FeCl2����
D����FeCl3������Һ����NaOH��Һ�п��Ʊ�Fe(OH)3����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������п�ͼ�ش�����(����ʱ������ʽ�е�M��E������Ӧ��Ԫ�ط��ű�ʾ)��
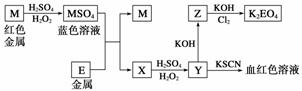
(1)д��M����ϡ�����H2O2���Һ�Ļ�ѧ����ʽ________________________��
(2)ijͬѧȡX����Һ���ữ�����KI������Һ����Ϊ��ɫ��д���������仯������ص����ӷ���ʽ________________________��________________________��
(3)д��Cl2��Z����ΪK2EO4�Ļ�ѧ����ʽ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ϊ Va ��0.05mol��L-1CH3COOH��Һ�м������ΪVb��0.05mol��L-1KOH��Һ�����й�ϵ�������ǣ�
A��Va>Vbʱ��c (CH3COOH) +c (CH3COO-)>c (K+��
B��Va=Vbʱ��c (CH3COOH) +c (H+)��c (OH-��
C��Va<Vbʱ��c (CH3COO-)>c (K+��> c (OH-��> c (H+��
D��Va��Vb�����ʱ��c (K+��+ c (H+) ��c (OH-��+ c (CH3COO-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�̨��������Һ
��pH���±���
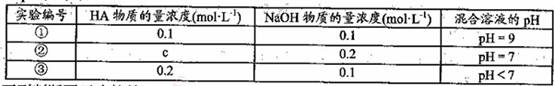
�����жϲ���ȷ����
A��HA�ĵ��뷽��ʽΪ��
B���������������ʵ������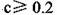
C������ʵ���У������Һ��
D������ʵ���У������Һ��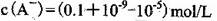
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com