������ͼ�ش����⣺
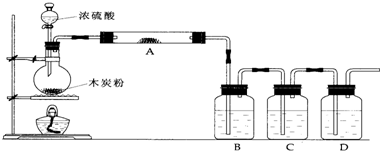
��1������װ���У��ڷ�Ӧǰ�����ƽ�����ƿ��ڼ��װ�õ������ԣ���۲첻�����Ե�����������ʲô�ķ���֤����װ�ò�©����
��
��Ӧǰ��ȼ�ƾ��ƣ�������ƿһС�������ƿB��C��D�г������ݣ�Ϩ��ƾ��ƣ�ƿB��C��D�е���Һ��������֤����װ�ò�©��
��Ӧǰ��ȼ�ƾ��ƣ�������ƿһС�������ƿB��C��D�г������ݣ�Ϩ��ƾ��ƣ�ƿB��C��D�е���Һ��������֤����װ�ò�©��
��2��д��Ũ�����ľ̿���ڼ��������·�����Ӧ�Ļ�ѧ����ʽ��
2H
2SO
4��Ũ��+C
CO
2��+2SO
2��+2H
2O
2H
2SO
4��Ũ��+C
CO
2��+2SO
2��+2H
2O
��3�������ͼ�е�װ�ü���������Ӧ��ȫ�����д������������ʾ��������Ӧ������Լ������Ƽ������ã�
A�м�����Լ���
��ˮ����ͭ
��ˮ����ͭ
��������
���� H2O
���� H2O
��
B�м�����Լ���
Ʒ����Һ
Ʒ����Һ
��������
���� SO2
���� SO2
��
C�м�����Լ���
��������KMnO4 ��Һ
��������KMnO4 ��Һ
�������dz���
SO2
SO2
���壮
D�м�����Լ���
����ʯ��ˮ
����ʯ��ˮ
��������
���� CO2
���� CO2
��
��4��ʵ��ʱ��C��Ӧ�۲쵽��������
����������ð������Һ��ɫ��dz��ƿ����������ɫ��������
����������ð������Һ��ɫ��dz��ƿ����������ɫ��������
��





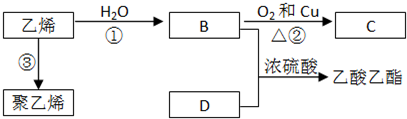
 ������ͼ�ش����⣺
������ͼ�ش����⣺











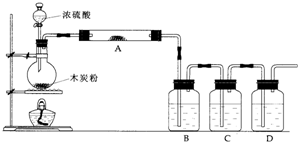 ������ͼ�ش����⣺
������ͼ�ش����⣺