
����Ŀ�������İ���ͭ����([Cu(NH3)4]SO4��H2O)������ɱ�����ýȾ�����ڼ��Զ�ͭ��Ҳ���������Һ����Ҫ�ɷ֣��ڹ�ҵ����;�㷺�������¸������ڿ����в��ȶ�������ʱ�����ֽ⡣ij��ѧ��ȤС����Cu�ۡ�3mol/L�����ᡢŨ��ˮ��10% NaOH��Һ��95%���Ҵ���Һ��0.500 mol/Lϡ���ᡢ0.500 mol/L��NaOH��Һ���ϳ������İ���ͭ���岢�ⶨ�䴿�ȡ�
I��CuSO4��Һ���Ʊ�
�ٳ�ȡ4gͭ�ۣ���A����������10���Ӳ����Ͻ��裬������ȴ��
�����������м���30mL 3mol/L�����ᣬ��A�й��������������У����Ȳ����Ͻ��衣
�۳��ȹ��˵���ɫ��Һ��
(1)A����������Ϊ________________________________��
(2)ijͬѧ��ʵ������1.5g��ͭ��ʣ�࣬��ͬѧ���Ƶõ�CuSO4��Һ������һ�������м���Ũ�����о�Ĥ���֣���ȴ�����ľ����к��а�ɫ��ĩ���Խ�����ԭ��__________________________________________��
II��������Ʊ�
�������Ʊ���CuSO4��Һ����ͼ��ʾ���в���
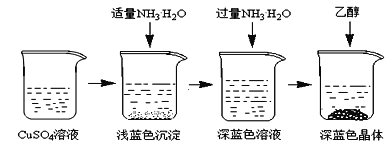
(3)��֪dz��ɫ�����ijɷ�ΪCu2(OH)2SO4����д�����ɴ˳��������ӷ�Ӧ����ʽ___________________��
(4)��������ʱ���ü����Ҵ��ķ�����������Ũ���ᾧ��ԭ����________________________��
III���������IJⶨ
��ȷ��ȡwg������������ˮ�ܽ���ע����ͼ��ʾ������ƿ����Ȼ����μ�������10%NaOH��Һ��ͨ��ˮ����������ƷҺ�еİ�ȫ����������������ˮ��ϴ�����ڱڣ���V1mL0.5mol/L���������Һ��ȫ���ա�ȡ�½���ƿ����0.5mol/L NaOH����Һ�ζ���ʣ��HCl(ѡ�ü�����ָʾ��)�����յ�ʱ����V2mLNaOH��Һ��
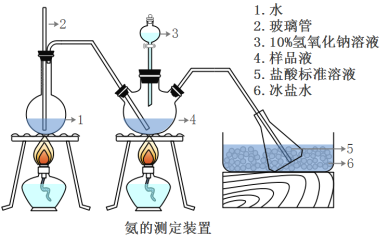
(5)Aװ���г������ܵ�����_________________����Ʒ�а������������ı���ʽ_______��
(6)����ʵ���������ʹ�������ⶨ���ƫ�ߵ�ԭ����____________________��
A���ζ�ʱδ��NaOH����Һ��ϴ�ζ��ܡ�
B������ʱ���ζ�ǰƽ�ӣ��ζ����ӡ�
C���ζ�������ѡ�÷�̪��ָʾ����
D��ȡ�½���ƿǰ��δ������ˮ��ϴ�������ƿ�еĵ�����ڡ�
���𰸡����� ��Ӧ�������������Ũ�������У�ϡ������Ũ��Ũ�������ˮ��ʹCuSO4![]() 5H2Oʧˮ���CuSO4 2Cu2++2NH3
5H2Oʧˮ���CuSO4 2Cu2++2NH3![]() H2O+SO42-=Cu2(OH)2SO4+2NH4+ �����İ���ͭ�����������ȷֽ� ƽ����ѹ����ֹ�����͵���
H2O+SO42-=Cu2(OH)2SO4+2NH4+ �����İ���ͭ�����������ȷֽ� ƽ����ѹ����ֹ�����͵��� ![]() BD
BD
��������
(1) ��������һ���������н�����
(2) ���Ƶõ�CuSO4��Һ������һ�������м���Ũ�����о�Ĥ���֣���ȴ�����ľ����к��а�ɫ��ĩ���ð�ɫ��ĩΪCuSO4��ԭ���Ƿ�Ӧ�������������Ũ�������У�ϡ������Ũ��Ũ�������ˮ��ʹCuSO4![]() 5H2Oʧˮ���CuSO4��
5H2Oʧˮ���CuSO4��
(3)�ɲ������̿�֪������ͭ��Һ��������ˮ��Ӧ����Cu2(OH)2SO4�������ݴ�д�����ӷ���ʽ��
(4) ������狀�ͭ������Ʊ������У������Ҵ����Խ��������İ���ͭ������ܽ�ȣ������ھ���������
(5) ��A��ѹ������ʱ����������Һ��������ʹAƿ��ѹ���ȶ���
���ݵζ���ȥ���������Ƶ����ʵ������Լ�����백��Ӧ����������ʵ�����������������İ��������ʵ������ټ����NH3�������ٷ�����
(6) ������Ʒ�а���������������ʽ![]() ������������V2ƫС����ʹ�������ⶨ���ƫ�ߣ��ݴ˷�����
������������V2ƫС����ʹ�������ⶨ���ƫ�ߣ��ݴ˷�����
(1) ��������һ���������н��У���ͭ��ת��ΪCuO��
�ʴ�Ϊ��������
(2) ���Ƶõ�CuSO4��Һ������һ�������м���Ũ�����о�Ĥ���֣���ȴ�����ľ����к��а�ɫ��ĩ���ð�ɫ��ĩΪCuSO4��ԭ���Ƿ�Ӧ�������������Ũ�������У�ϡ������Ũ��Ũ�������ˮ��ʹCuSO4![]() 5H2Oʧˮ���CuSO4��
5H2Oʧˮ���CuSO4��
�ʴ�Ϊ����Ӧ�������������Ũ�������У�ϡ������Ũ��Ũ�������ˮ��ʹCuSO4![]() 5H2Oʧˮ���CuSO4��
5H2Oʧˮ���CuSO4��
(3)�ɲ������̿�֪������ͭ��Һ��������ˮ��Ӧ����Cu2(OH)2SO4���������ӷ�Ӧ����ʽΪ��2Cu2++2NH3![]() H2O+SO42-=Cu2(OH)2SO4+2NH4+��
H2O+SO42-=Cu2(OH)2SO4+2NH4+��
�ʴ�Ϊ��2Cu2++2NH3![]() H2O+SO42-=Cu2(OH)2SO4+2NH4+��
H2O+SO42-=Cu2(OH)2SO4+2NH4+��
(4) �������Ϣ��֪�������İ���ͭ�����������ȷֽ⣬�������Ҵ����Խ��������İ���ͭ������ܽ�ȣ������ھ���������
�ʴ�Ϊ�������İ���ͭ�����������ȷֽ���
(5) ��A��ѹ������ʱ������������Һ��������ʹAƿ��ѹ���ȶ������������ܵ�������ƽ����ѹ����ֹ�����͵�����
�ʴ�Ϊ��ƽ����ѹ����ֹ�����͵�����
��������ݿ�֪�������İ��������ʵ���Ϊ(0.5V1-0.5V2)��10-3mol��
������Ʒ�а�����������Ϊ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
(6)������Ʒ�а���������������ʽ![]() ����ʵ���������V2ƫС����ʹ�������ⶨ���ƫ�ߣ�
����ʵ���������V2ƫС����ʹ�������ⶨ���ƫ�ߣ�
A���ζ�ʱδ��NaOH����Һ��ϴ�ζ��ܣ�����������ҺŨ��ƫС�����ĵ����V2ƫ����ʹ�������ⶨ���ƫ�ͣ����������⣻
B������ʱ���ζ�ǰƽ�ӣ��ζ����ӣ����ĵ����V2ƫС����ʹ�������ⶨ���ƫ�ߣ��������⣻
C���ζ�������ѡ�÷�̪��ָʾ�������ĵ����V2ƫ����ʹ�������ⶨ���ƫ�ͣ����������⣻
D��ȡ�½���ƿǰ��δ������ˮ��ϴ�������ƿ�еĵ�����ڣ����ĵ����V2ƫС����ʹ�������ⶨ���ƫ�ߣ��������⡣
�ʴ�Ϊ��BD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ں����ܱ������з�����Ӧ2SiHCl3(g)SiH2Cl2(g)+SiCl4(g)����323K��343KʱSiHCl3��ת������ʱ��仯�Ľ����ͼ��ʾ������˵����ȷ����(����)

A. 323 Kʱ����С������������SiHCl3��ת����
B. a��b���Ӧ�ķ�Ӧ���ʴ�С��ϵ��v(a)��v(b)
C. 343 Kʱ��������Ӧ�Ļ�ѧƽ�ⳣ��ԼΪ0.02
D. 2SiHCl3(g)SiH2Cl2(g)+SiCl4(g)������ӦΪ���ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������β���ﺬ�е�NO������������ȼ��ȼ�յĸ�����������������Ӧ��N2(g)��O2(g��![]() 2NO(g����H ��0����֪�÷�Ӧ��240����ƽ�ⳣ��K��64��10��4����ش�
2NO(g����H ��0����֪�÷�Ӧ��240����ƽ�ⳣ��K��64��10��4����ش�
��1��ij�¶��£���2 L���ܱ������г���N2��O2��1 mol��5���Ӻ�O2�����ʵ���Ϊ0.5 mol����N2�ķ�Ӧ����Ϊ_____________________��
��2���ٶ��÷�Ӧ���ں��������½��У��жϸ÷�Ӧ�ﵽƽ��ı�־________ ��
A������1 mol N2ͬʱ����1 mol O2
B����������ܶȲ���
C���������ƽ����Է�����������
D��2v��(N2)��v��(NO)
��3����N2��O2�Ļ�����������º����ܱ������У���ͼ�仯������ȷ����____________(����ĸ���)��

��4������º��ݵ��ܱ������г�������ʵ�����N2��O2���ﵽƽ��״̬���������г���һ����NO�����´ﵽ��ѧƽ��״̬����ԭƽ��״̬��ȣ���ʱƽ��������NO���������________��(��������С�����䡱)
��5�����¶��£�ijʱ�̲��������N2��O2��NO��Ũ�ȷֱ�Ϊ2.5��10��1 mol��L��1��4.0��10��2 mol��L��1��3.0��10��3 mol��L��1����ʱ��Ӧ___________________(����ڻ�ѧƽ��״̬������������Ӧ������С������淴Ӧ������С�)��������________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ������84����Һ��ͨ��ϡ��100��(���֮��)��ʹ�á���ش��������⣺
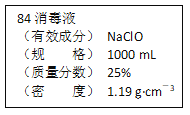
(1)����84����Һ�������ʵ���Ũ��ԼΪ_____mol��L��1��
(2)ȡ����������ĸ�����Һʱ�������������л�����ȡ����Ķ��ٶ��仯����________(����ĸ)��
A����Һ��NaClO�����ʵ��� B����Һ��Ũ��
C����Һ��NaClO��Ħ������ D����Һ���ܶ�
(3)��ͬѧ���ĸ���84����Һ�����䷽������NaClO��������480 mL��NaClO��������Ϊ25%������Һ���ش��������⡣
����ͼ��ʾ�������У���Щ�Dz���Ҫ������������Һ����Ҫ��������_______

����Ҫ����NaClO���������Ϊ_______ g
(4)��84����Һ����ϡ������ʹ�ÿ���ǿ����������ij����С����Ա��98%(�ܶ�Ϊ1.84 g��cm��3)��Ũ��������200 mL 2.3 mol��L��1��ϡ����������ǿ��84����Һ��������������
�������Ƶ�ϡ�����У�H�������ʵ���Ũ��Ϊ________mol��L��1��
������Ũ��������Ϊ________ mL��
���������Ƶ�ϡ����Ũ��ƫС�������п��ܵ�ԭ���������ȷ����_______��
A������ǰ������ƿ������������ˮ B����ȡŨ����ʱ������Һ��İ�Һ��
C��δ��ȴ������ת��������ƿ���� D������ʱ��������Һ�İ�Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ϊ�����Դ���Ź㷺��Ӧ��ǰ����������Ȼ���Ʊ��������������¡�

��ش��������⣺
I��ת��������Ȼ��ѹ����������30��ʱ����T��F�������£����Ի������������ʾ��ͼ���¡�
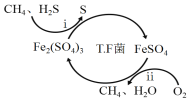
(1)����i�����ӷ�Ӧ����ʽΪ_________________________________________��
(2)��֪��
��Fe3+��pH=l.9ʱ��ʼ������pH=3.2ʱ������ȫ��
��30��ʱ����T.F�������£���ͬpH��FeSO4��Һ��Fe2+�������������±���
pH | 0.7 | 1.1 | 1.5 | 1.9 | 2.3 | 2.7 |
Fe2+����������/g��L-1��h-1 | 4.5 | 5.3 | 6.2 | 6.8 | 7.0 | 6.6 |
��ת�������У������ϱ���ѡ�����pH��Χ��_______<pH<_______������ѡ���ԭ���ǣ�_______________________________________________��
������ת�����ڴ����������£�ˮ������CH4�����������ͼ�ش����⡣
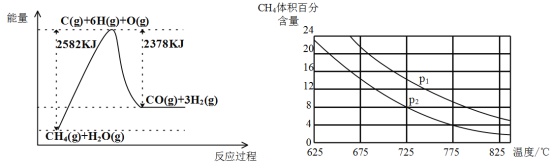
(3)�ٸù��̵��Ȼ�ѧ����ʽ��__________________________________________��
�ڱȽ�ѹǿP1��p2�Ĵ�С��ϵ��P1 _________ P2(ѡ����>����<������=��)��
����һ���¶Ⱥ�һ��ѹǿ�µ�����ɱ���ܱ������г���1molCH4��1mol��ˮ������ַ�Ӧ��ƽ������ʼʱ��������ܶ���ƽ��ʱ������ܶȵ�1.4��������ʱ���������Ϊ2L,��÷�Ӧ��ƽ�ⳣ��Ϊ______________(�������2λ��Ч����)��
����CO�任��500��ʱ��CO��һ����ˮ��Ӧ����CO2��H2��
����H2�ᴿ����CO2��H2����õ�H2�Ĺ�����ʾ��ͼ
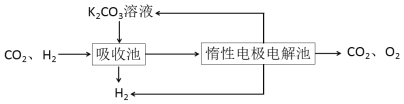
(4)���ճ��з�����Ӧ�����ӷ���ʽ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ϻ�ʵ�ʲ����ڹ�ҵ�������ǣ� ��
A.Na��![]() ��ȼ����NaClB.Ũ
��ȼ����NaClB.Ũ![]() ��NaCl��Ӧ��HCl
��NaCl��Ӧ��HCl
C.![]() ��ʯ����������Ư�۾�D.H2��
��ʯ����������Ư�۾�D.H2��![]() ��ֻ�Ϲ�����HCl
��ֻ�Ϲ�����HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�������ͬ���칹����Ŀ���ٵ���
A. ����B. C5H11Cl
C. ��ϩD. �������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ǰ�����ڵ�A��B��C��D����Ԫ�������ڱ��о���Ԫ��X�������ڡ���֪Ԫ��X���������Ļ�ѧʽΪX2O5��B��Dͬ������BԪ�ص�ԭ�Ӱ뾶��ͬ��Ԫ������С�ģ�C������������Ӧ��ˮ������ǿ�ᡣ
��1��DԪ�ػ�̬ԭ�ӵ���Χ�����Ų�ʽΪ____________________��
��2��A��C��X����Ԫ��ԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ________________������Ӧ��Ԫ�ط������𣩡�
��3��B��X��D�⻯��ķе��ɸߵ��͵�˳��Ϊ_______________������Ӧ�Ļ�ѧʽ���𣩡�
��4��CԪ�ص�ԭ�ӿ��γɶ������ӣ����Ʋ������������幹�ͣ�CΪ��ĸ������̼Ԫ�أ���
�� | CO32- | CO42- |
���幹������ | _______________ | _______________ |
��5��Ԫ��B��һ���⻯��B2H4������Ҫ����;���й�B2H4��˵����ȷ����_______��
A��B2H4���Ӽ���γ���� B��Bԭ����sp3�ӻ�
C��B2H4�����к���5���Ҽ���1���м� D��B2H4�����ΪҺ̬ʱ�ƻ����ۼ�
��6��EԪ�غ�DԪ����ͬһ���ڣ�����VIII�壬�۲������������ӣ�E(OH)2Ϊ�������������Ũ��ǿ����Һ�п��γ�E(OH)42-��д��E(OH)2��ʽ����ĵ��뷽��ʽ___________________��
��7��FԪ�ػ�̬ԭ��M������5�ԳɶԵ��ӣ�F�γɵĵ����Цġ��á�������ͬ�������壬���־�������ͼ��ʾ����Fԭ�ӵ���λ��֮��Ϊ___________���ġ��á������־����ı߳�֮��Ϊ_____________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾΪԪ�����ڱ��м�Ԫ�ؿ�ͼ��������39.10����ʾ����________��
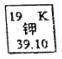
ij��ѧ��ȤС������ȥ16g�����Ȼ����л��е������Ȼ��ƺ�����þ���ʣ����ʵ�鷽�����£���֪������NaCl���ܽ��Ϊ36g/100g![]() O��
O��

��1����ˮ�ܽ�ʱ���ʺϵ�ȡˮ��Ϊ________
A. 20mL B. 45mL C. 75mL D. 100mL
��2��Ϊ�ӿ��ܽ����ʣ��ɲ�ȡ�ķ�����________��________����д2�֣�
��3�����μ���Ĺ����Լ�Ϊ________��________��________��
��4����ҺA�м����������Ϊ________��������Ҳ�ӹ����������õ��Ȼ��ƵĴ���________��������������û������Ӱ��
��5���������������________������II��������________�����ֲ����ж��õ��IJ���������________��
��6�����õ��Ĺ����Ȼ�����ԭ���������е��Ȼ���������ȣ����________������������������С����������������
��7������A���������εĻ�ѧʽΪ___________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com