
����Ҫ��д�����з�Ӧ���Ȼ�ѧ����ʽ
(1)һ����������������Ӧ�����Ȼ�������,������1mol���ȼ�ʱ�ų�91.5kJ������________________________________________________________.
(2)ij��ѧ��Ӧ�������仯��ͼ��ʾ,�÷�Ӧ���Ȼ�ѧ����ʽ��(��Ӧ����abc��ʾ)
_____________________________________________.
(3)ij��Ӧ��ƽ�ⳣ��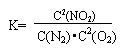 �����1molN2��ȫ��Ӧ,Ҫ��������68kJ.д���÷�Ӧ���Ȼ�ѧ����ʽ______________________________________________.
�����1molN2��ȫ��Ӧ,Ҫ��������68kJ.д���÷�Ӧ���Ȼ�ѧ����ʽ______________________________________________.
(4)ʵ���в���ֱ�Ӳ��ʯī���������ɼ��鷴Ӧ�ķ�Ӧ��,���ɲ�����顢ʯī������ȼ�յķ�Ӧ��:
CH4(g)+2O2(g)=CO2(g)+2H2O(l)����H1=-890.3kJ/mol
C(ʯī,s)+O2(g)=CO2(g) ����H2="-393.5" kJ/mol
H2(g)+1/2O2(g)=H2O(l) ����H3="-285.8" kJ/moL
����ʯī��������Ӧ���ɼ�����Ȼ�ѧ��Ӧ����ʽΪ__________________________________________.

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
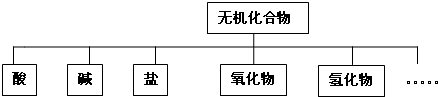
| ������� | �� | �� | �� | ������ | �⻯�� |
| ��ѧʽ | ��HCl �� H2SO4��HNO3 H2SO4��HNO3 |
�� NaOH��KOH NaOH��KOH ��Ba��OH��2 |
��Na2CO3 �� NaNO3��KNO3��K2SO4��Na2SO4 NaNO3��KNO3��K2SO4��Na2SO4 |
��CO2 ��Na2O |
��NH3 ��H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�챱���и߶�����3�·��¿���ѧ�Ծ� ���ͣ������
����Ҫ��д�����з�Ӧ���Ȼ�ѧ����ʽ
(1)һ����������������Ӧ�����Ȼ�������,������1mol���ȼ�ʱ�ų�91.5kJ������________________________________________________________.
(2)ij��ѧ��Ӧ�������仯��ͼ��ʾ,�÷�Ӧ���Ȼ�ѧ����ʽ��(��Ӧ����abc��ʾ)
_____________________________________________.

(3)ij��Ӧ��ƽ�ⳣ�� �����1molN2 ��ȫ��Ӧ,Ҫ��������68kJ.д���÷�Ӧ���Ȼ�ѧ����ʽ______________________________________________.
�����1molN2 ��ȫ��Ӧ,Ҫ��������68kJ.д���÷�Ӧ���Ȼ�ѧ����ʽ______________________________________________.
(4)ʵ���в���ֱ�Ӳ��ʯī���������ɼ��鷴Ӧ�ķ�Ӧ��,���ɲ�����顢ʯī������ȼ�յķ�Ӧ��:
CH4(g)+2O2(g)=CO2(g)+2H2O(l)����H1=-890.3kJ/mol
C(ʯī,s)+O2(g)=CO2(g) ����H2=-393.5 kJ/mol
H2(g)+1/2O2(g)=H2O(l) ����H3=-285.8 kJ/moL
����ʯī��������Ӧ���ɼ�����Ȼ�ѧ��Ӧ����ʽΪ__________________________________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ҫ��д�����з�Ӧ���Ȼ�ѧ����ʽ
(1)һ����������������Ӧ�����Ȼ�������,������1mol���ȼ�ʱ�ų�91.5kJ������________________________________________________________.
(2)ij��ѧ��Ӧ�������仯��ͼ��ʾ,�÷�Ӧ���Ȼ�ѧ����ʽ��(��Ӧ����abc��ʾ)
_____________________________________________.
(3)ij��Ӧ��ƽ�ⳣ�������1molN2 ��ȫ��Ӧ,Ҫ��������68kJ.д���÷�Ӧ���Ȼ�ѧ����ʽ______________________________________________.
(4)ʵ���в���ֱ�Ӳ��ʯī���������ɼ��鷴Ӧ�ķ�Ӧ��,���ɲ�����顢ʯī������ȼ�յķ�Ӧ��:
CH4(g)+2O2(g)=CO2(g)+2H2O(l)����H1=-890.3kJ/mol
C(ʯī,s)+O2(g)=CO2(g)����H2=-393.5 kJ/mol
H2(g)+1/2O2(g)=H2O(l)����H3=-285.8kJ/moL
����ʯī��������Ӧ���ɼ�����Ȼ�ѧ��Ӧ����ʽΪ__________________________________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ��Ϫ�и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��12�֣�����Ҫ����գ�
��1������Ҫ��д�����з�Ӧ�Ļ�ѧ����ʽ��
����ˮ�μӵ��û���Ӧ������������
����ˮ�μӵ�������ԭ��Ӧ��ˮ�������������ǻ�ԭ�� ��
����ˮ�μӵ�������ԭ��Ӧ����ˮ�Ȳ����������ֲ��ǻ�ԭ�� ��
����ˮ�μӵĸ��ֽⷴӦ ��
��2������ɫ�Լ���˫��ˮ����Ϊ��ҵ�����������������ɿ�ҵ��Һ�е��軯���KCN���Ļ�ѧ����ʽΪ��KCN+H2O2+H2O=A+NH3��
��������A�Ļ�ѧʽΪ ��
���ڱ�״������0.448L�������ɣ���ת�Ƶĵ�����Ϊ ��
�۷�Ӧ�б�������Ԫ��Ϊ ��
��3��ͬ��ͬѹ�£�ͬ�����NH3��H2S���������� ��ͬ������NH3��H2S��������� ��������������ԭ�ڸ�����ȣ�NH3��H2S�����ʵ������� ��
��4������״���µ�NH3��g��VL����ˮ�У��õ��ܶ�Ϊb g��cm-3�İ�ˮa g����ʱ���ʵ���Ũ��Ϊc mol��L-1��������ˮ�е�NH3��g�������V�� L����a��b��c�ȱ�ʾ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com